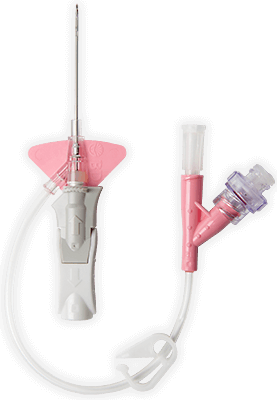
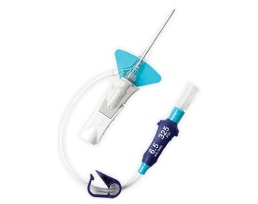
Zamknięty system cewnika dożylnego BD Nexiva™ Diffusics™ zbudowany jest w oparciu o zamknięty system cewnika dożylnego BD Nexiva™ z dodatkowym bonusem w postaci dyfuzyjnej końcówki cewnika, aby sprostać wyzwaniom związanym z tomografią komputerową (TK) podczas wykonywania procedury wstrzykiwania ciśnieniowego, bez zwiększania ryzyka powikłań dożylnych.1,2
Cewnik dożylny BD Nexiva™ Diffusics™ w systemie zamkniętym
Skuteczność w każdej sytuacji


- Przegląd
- EIFU i Zasoby
Mniejsze ryzyko infiltracji/wynaczynienia
W końcówce cewnika znajdują się liczne otwory w kształcie kropli, rozpraszające przepływ i osłabiające działanie sił, które mogłyby przemieścić cewnik w żyle nawet o 67%.*3
Większa wydajność
Cewnik dożylny pozwala na uzyskanie większego przepływu przy mniejszym rozmiarze cewnika (od 22 do 24 G)*
Potwierdzenie wprowadzenia do naczynia
Technologia BD Instaflash™ obejmuje igły z nacięciami, które są w stanie zapewnić większą skuteczność pierwszego wprowadzenia cewnika, ograniczając jednocześnie konieczność bolesnego ponownego cewnikowania.
Czas utrzymania cewnika
Zastrzeżony prawnie materiał biologiczny BD Vialon™ zmiękcza się w żyle nawet o 70%, umożliwiając dłuższe utrzymywanie cewnika i zmniejszając ryzyko mechanicznego zapalenia żył nawet o 50%.4†
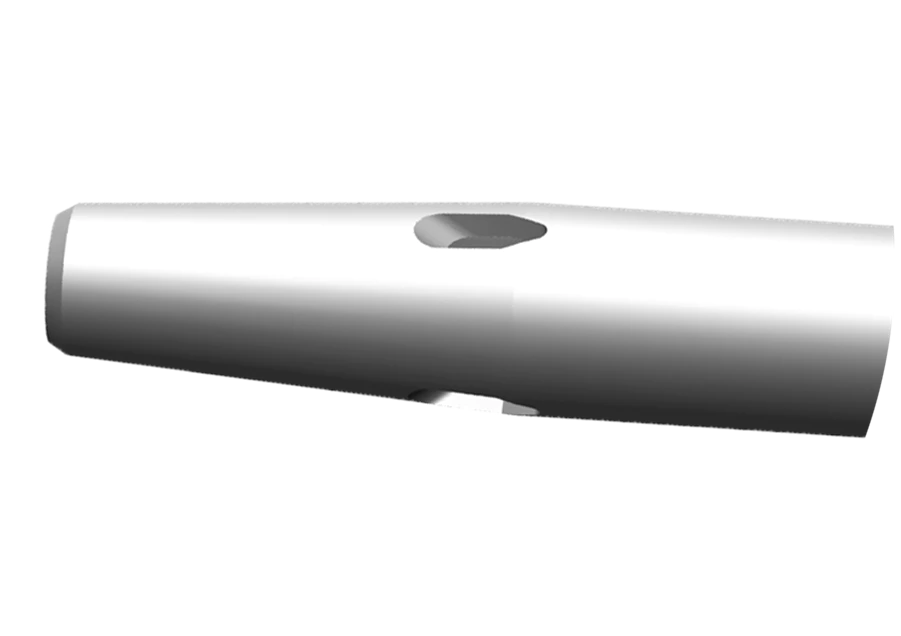
Dyfuzyjna końcówka cewnika
- Znajdują się na niej liczne otwory w kształcie kropli, rozpraszające przepływ i osłabiające działanie sił, które mogłyby przemieścić cewnik w żyle podczas badania TK z kontrastem nawet o 67%.
- Umożliwia większy przepływ przy mniejszym rozmiarze cewnika (od 22 do 24 G) na potrzeby wstrzykiwania automatycznego.*
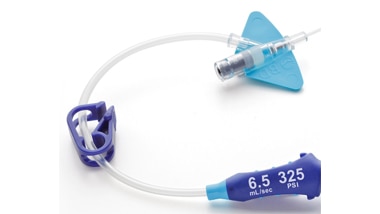
Zintegrowany system
- System jest wyposażony w zintegrowany cewnik i zestaw przedłużający, obsługujący wstrzykiwacz ciśnieniowy o ustawieniu 325 psi.
- Zintegrowany, zamknięty system, odpowiadający na typowe problemy żylne podczas wstrzykiwania automatycznego na potrzeby badania TK.*
- Wymaga mniejszej liczby urządzeń dodatkowych, ograniczając w ten sposób konieczność manipulacji, które mogłyby doprowadzić do zanieczyszczenia przez dotyk oraz przypadkowego rozłączenia.6,7*
Platforma stabilizująca
- System BD Nexiva Diffusics jest wyposażony we wbudowaną platformę stabilizującą.
- Dzięki wbudowanej platformie system minimalizuje ruchy cewnika w żyle i poza nią.2**

Technologia igieł Instaflash™ firmy BD
- Natychmiastowe potwierdzenie wprowadzenia cewnika do naczynia.
- Szybka wizualizacja krwi w cewniku może poprawić skuteczność wprowadzania, a tym samym ograniczyć konieczność powtarzania cewnikowania.

Materiał cewnika BD Vialon™
- Klinicznie udowodnione utrzymywanie cewnika nawet przez 144 godziny.1
- Ryzyko mechanicznego zapalenia żył zmniejszone nawet o 50%4†

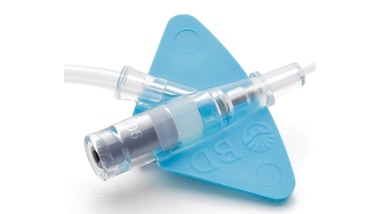
Adapter luer
- System jest wyposażony w adapter luer ze wskazaniem maksymalnego przepływu i wartości ciśnienia dla wstrzykiwań automatycznych.
Większa stabilizacja cewnika
Stabilizowanie cewnika jest uznawane za interwencję mającą zmniejszyć ryzyko powikłań. Może przynieść korzyści w postaci zapobiegania zakażeniom krwi związanym z cewnikiem (CRBSI).6,8
Zintegrowana konfiguracja
Infusion Nurses Society (INS) zaleca ograniczenie stosowania urządzeń dodatkowych, aby ograniczyć konieczność dodatkowych manipulacji oraz zmniejszyć ryzyko zanieczyszczenia i rozłączenia cewnika.6
Większe bezpieczeństwo klinicysty
Fabrycznie zmontowany system cewnika dożylnego BD Nexiva Diffusics zmniejsza ryzyko kontaktu z krwią podczas wprowadzania cewnika o 98%.2*
BD Nexiva™ Diffusics™ — techniki używania
Uwaga: niektóre produkty, usługi oraz funkcje produktów i usług mogą być niedostępne w danym regionie. Skontaktuj się z lokalnym przedstawicielem firmy BD.
* W porównaniu z cewnikiem dożylnym bez końcówki dyfuzyjnej.
† W porównaniu z cewnikiem z kopolimeru fluorowego etylenu/propylenu (FEP)
** W porównaniu z cewnikiem B. Braun Introcan Safety® z urządzeniem stabilizującym Bard Statlock® IV Ultra.
Odniesienia
- Gonzalez Lopez J, Arribi Vilela A, Fernandez Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Tamura N, Ave S, Hagimoto K, et al. Unfavorable peripheral intravenous catheter replacements an be reduced using an integrated closed intravenous catheter system. J Vasc Access. 2014;15(4):257-263.)
- Dane z badań własnych firmy BD. BD Protocol 10765 – (67% claim) - 14 czerwca 2011 r.; BD Protocol 12163 – Nexiva Diffusics Catheter Stability Rev 1 (Extravasation) - 16 lipca 2012 r.; BD Protocol 11226 – Arcturus Cath Motion Test Report (Extravasation) - 20 września 2011 r.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheter. Annals of Internal Medicine. 1991;114:845-854.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.)
- Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. 2021, Infusion Therapy Standards of Practice, 8th Edition. Journal of Infusion Nursing. Section 6 (S108).
- Alexander M, Corrigan A. Gorski L, et al. Infusion nursing: an evidence based approach. 3rd ed. St. Louis, MO: Saunders Elsevier; 2010:410.
- O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16.
BD-37378 (09/2021)