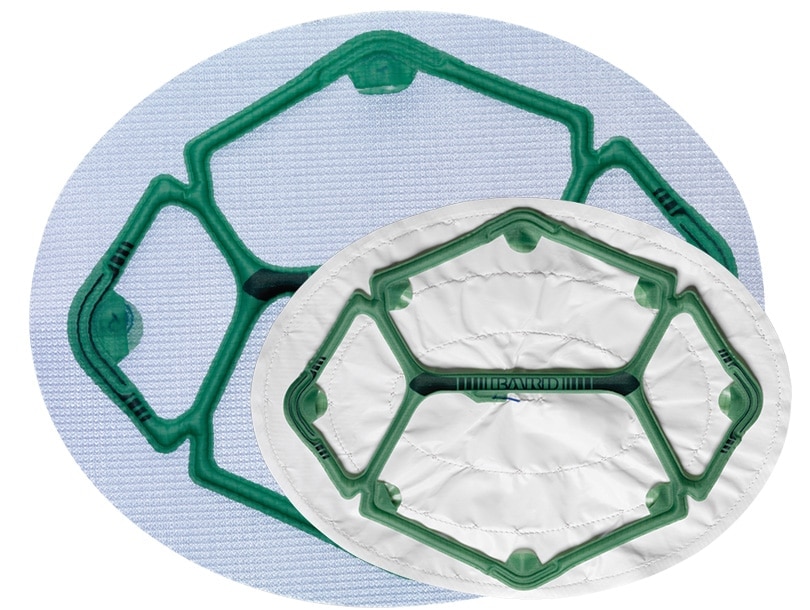
Ventralight™ ST Mesh or Composix™ L/P Mesh with a pre-attached low-profile balloon to help facilitate deployment, placement and positioning in laparoscopic ventral hernia repair.
Maille Ventralight™ ST avec système de positionnement Echo PS™, cercle, 6 po (15,2 cm)
- Présentation
- Spécifications
- Mode d’emploi électronique et ressources
- FAQ
- Produits connexes
- Notifications sur les produits
GTIN - null
00801741201974
1
Quantité
1/cs
Dimensions
Cercle de 6 po (15,2 cm)
GTIN
| GTIN - null | 00801741201974 | 1 |
Emballage
| Quantité | 1/cs |
Spécifications du produit
| Dimensions | Cercle de 6 po (15,2 cm) |
Si vous êtes un patient ou un utilisateur final, vous pouvez nous contacter vous-même, ou vous pouvez demander à votre soignant ou à votre médecin de le faire pour vous. Pour nous aider à traiter vos
informations rapidement et efficacement, veuillez contacter notre équipe chargée des réclamations clients.
Pour faciliter notre enquête, veuillez inclure les informations suivantes dans votre rapport:
- Nom du produit et/ou numéro de catalogue
- Numéro de lot ou numéro de série
- Blessures et/ou préjudices?
- Quel est le problème que vous avez rencontré?
- Est-ce-que l'échantillon réel ou le représentant de l'échantillon est disponible? (Si possible, veuillez envoyer l'échantillon concerné)
- Nom et numéro de téléphone de la personne à contacter
1 When compared to positioning with transfascial sutures in a preclinical study.
Date on file at C. R. Bard, Inc. Results may not correlate to performance in humans.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias. Composix™ L/P Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias and chest wall defects. The Echo PS™ Positioning System is intended to be used to facilitate the delivery of soft tissue prostheses during laparoscopic hernia repair.
CONTRAINDICATIONS
Literature reports there is a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
Ventralight™ ST Mesh/Composix™ L/P Mesh is the only permanent implant component of the device. The inflation adapter and syringe are to be kept external to the patient and discarded after use. The Echo PS™ Positioning System (including the balloon, all connectors, and inflation tube) is to be removed from the patient and appropriately discarded as it is not part of the permanent implant.
The Echo PS™ Positioning System should not be used with any other hernia prosthesis aside from those with which it comes pre-attached/packaged.
PRECAUTIONS
Do not trim the mesh. This will affect the interface between the mesh and positioning system.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use
.
