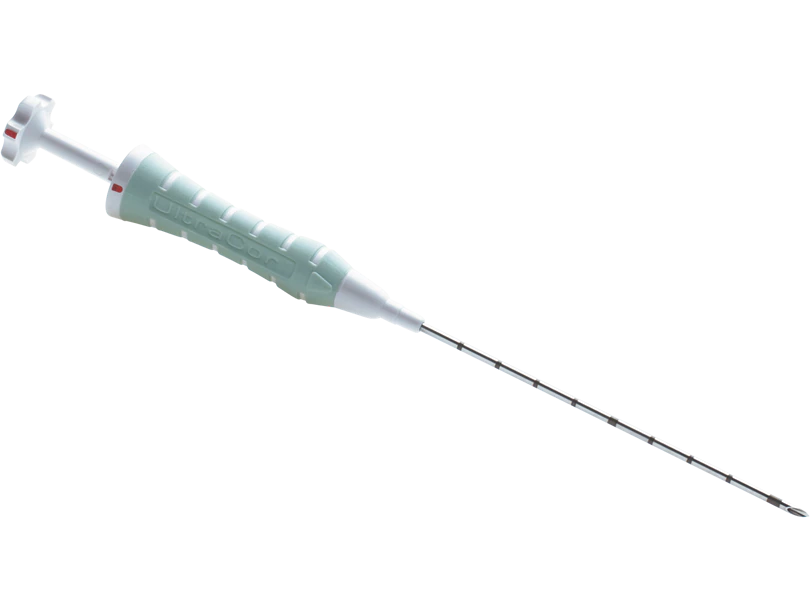
The Gel Mark UltraCor™ Breast Tissue Marker features a 14G rigid needle with a beveled tip and deploys 4 polylactic acid/polyglycolic acid pellets and one radiopaque marker. The polylactic acid/polyglycolic acid pellets provide approximately 6 weeks of enhanced ultrasound visibility.
For sales inquiries, technical support and customer support complete the following form:

- Overview
- Products & Accessories
- EIFU & Resources


Marker Shapes that Celebrate Her
With each purchase of the Heart, Venus or Ring breast tissue marker shapes, BD will contribute $1 to the American Cancer Society™ in honor of breast cancer patients.*

Gel Mark UltraCor™ Breast Tissue Marker PLA/PGA pellets provide approximately 6 weeks of ultrasound enhanced visibility and are essentially resorbed in approximately 12 weeks.
Designed for accuracy and visibility
* Applies to specially marked BD breast tissue marker products purchased from BD. The American Cancer Society™ does not endorse any service or product. Visit bd.com/icoh for details. Valid through September 30.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-110491
To view ordering and compatibility information for all BD Biopsy breast tissue markers, download the marker ordering sheet.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-19526
BD-19526
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
To view ordering and compatibility information for all BD Biopsy breast tissue markers, download the marker ordering sheet.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-19526
