Services
BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.

BD Expands Its Services Capabilities With ZebraSci
ZebraSci brings a rich history rooted in vision inspection, equipment design, and automation now applied to primary containers and complex devices.
RIGHT SOLUTIONS, RIGHT TIME
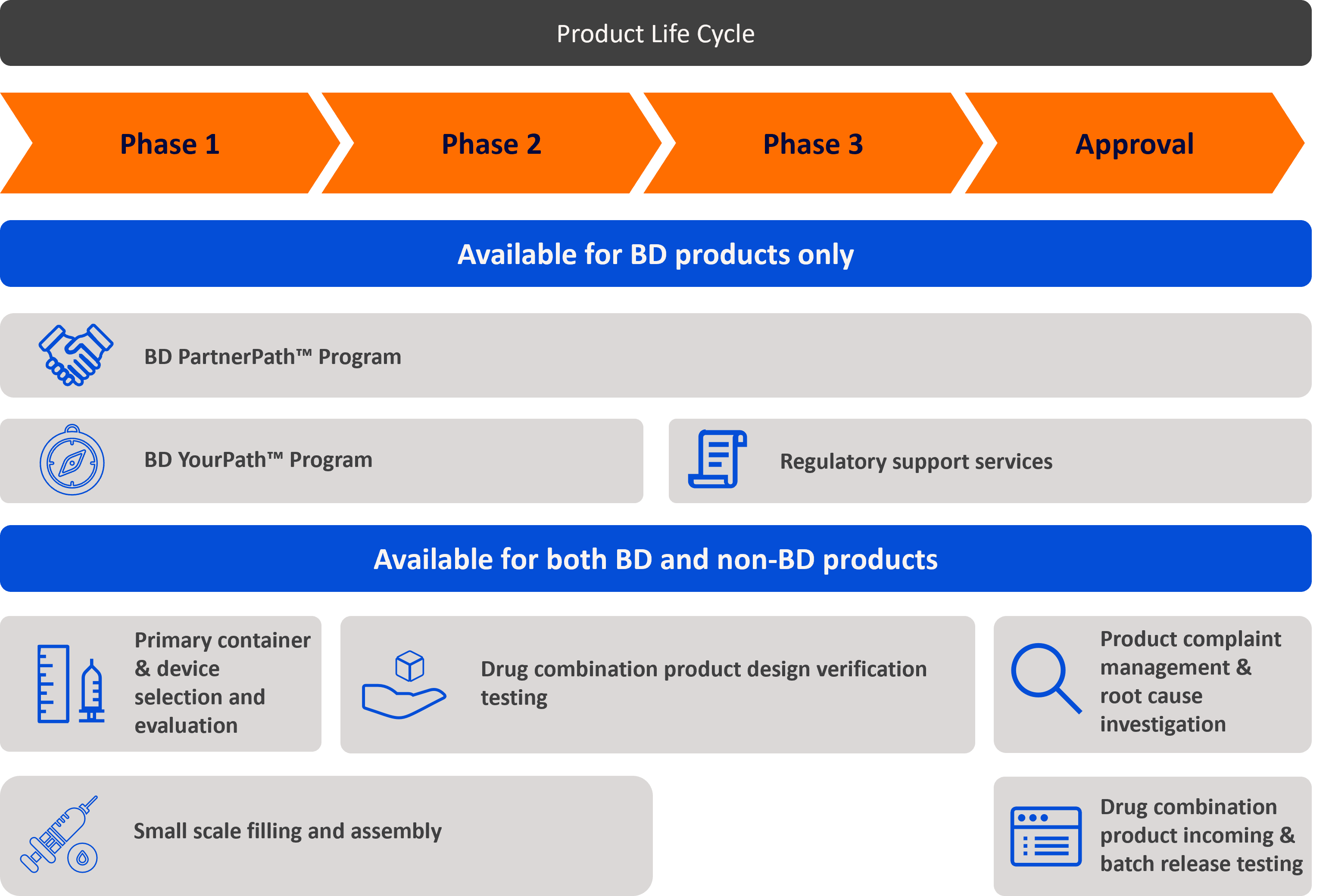
No matter where you are in the process of combination product development, BD can help you reach your goals.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
BD PartnerPath™ Program
Support your journey to market with streamlined access to preconfigured BD product sets and data packages (available for select products and product systems)
BD YourPath™ Program
Navigate the world of combination products with flexible support
Regulatory support services
Expand your reach into target markets with global experience and expertise in meeting combination product regulatory requirements
Primary container & device selection and evaluation
Available for both BD and non-BD products
For phase 1
Drug combination product design verification testing
De-risk your drug combination product solution through Good Manufacturing Practices (GMP) laboratory testing:
- Product engineering and device testing
- Primary container and drug interaction
- Combination product performance
- Container closure integrity (CCI) testing
Product complaint management & root cause investigation
Available for both BD and non-BD products
For approval
Small scale filling and assembly
Access to Good Manufacturing Practices (GMP) not-for-human-use filling, assembly and labeling services to support test quantities
Drug combination product incoming & batch release testing
Available for both BD and non-BD products
For approval
Efficient services to develop, de-risk
and accelerate delivery of your product
Each of our services is delivered intentionally and carefully to de-risk your development journey and accelerate the launch of your drug combination product on the market
-

Flexible offer
Our goal : offer services tailored to your needs
-

Access to scientific testing experts
Our goal : provide rapid and accurate answers to your questions
-

World-class container selection and validation
Our goal : develop optimal solutions for your primary container and secondary device
-

Combination product knowledge and testing capabilities
Our goal : ensure component and system performance consistency
-

Customizable protocols
Our goal : deliver relevant results addressing your specific needs
-

GMP-compliant labs with state-of-the-art equipment
Our goal : provide you with high-quality test results that can be leveraged in regulatory filings
Develop. De-risk. Deliver.
BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.