BD PartnerPath™ Program
BD PartnerPath™ Program supports the development of combination products, with quick access to small quantities of preconfigured drug delivery systems and supporting documentation.

- A Path to Market, Simplified
- Where Expertise Meets Simplicity
- BD PartnerPath™ For Expertise And Practical Support
Combination Product Development is Inherently Challenging
Let s face those challenges together
Developing a combination product is inherently challenging, and every step in the process can present hurdles.
From acquiring data and selecting the ideal drug delivery system, to formulating your regulatory strategy and conducting clinical trials, the road to commercialization can be fraught with challenges.
Unexpected challenges can delay your launch, increase costs, negatively impact profitability1 and impact the viability of your project.
The BD PartnerPath™ Program can help. The Program offers solutions across combination product development journey including:
-

Device identification2,3
-

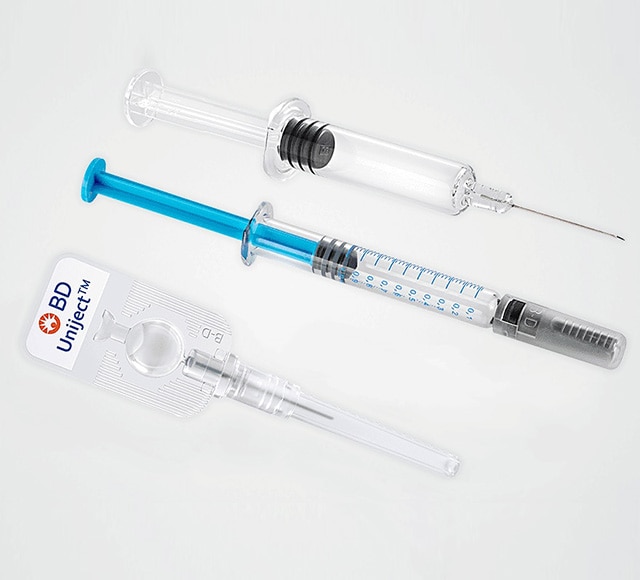
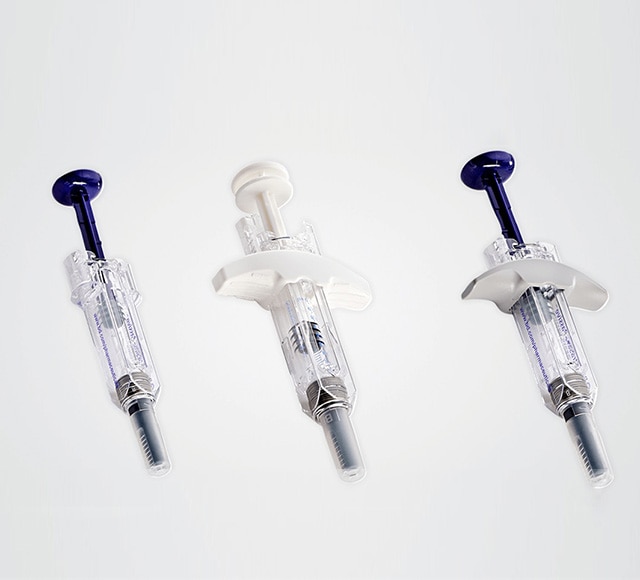
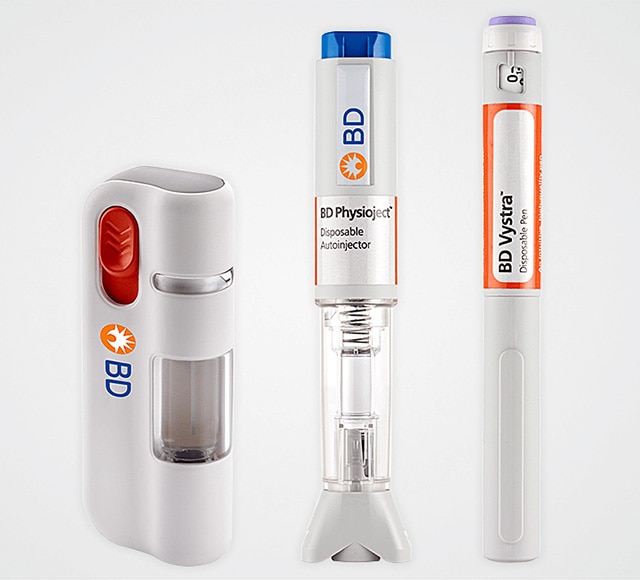
Quick access to small quantities
of preconfigured BD drug delivery systems
-

Drug compatibility4
-

Operational, performance and integration feasibility1
-

Regulatory registration5,6,7
Support in a Competitive Market,
Whatever Your Drug Development Experience
Solve combination product development challenges together: Leverage the BD PartnerPath™ Program and BD expertise
Whether you are new to combination product development, or you’ve been through it before, you will inevitably face challenges. Some challenges you will anticipate, others you will not.
For various reasons, you may be tempted to go it alone.
However, those development challenges could delay your launch target, creating additional problems along the way.1
But you do not have to go through it alone.
Everyday BD PartnerPath™ Program experts, with their deep industry experience, support combination product developers just like you. Why should you struggle alone, when you can take advantage of our services?
Engage in the BD PartnerPath™ Program and we can help you to:

Strategize on development processes

Develop a regulatory strategy

Human Factor studies

Focus your resources on your core expertises
Meet Combination Product Development Challenges with BD PartnerPath™ Program
To help accelerate bringing your drug combination product to market.
This is a competitive environment.
We understand the pressure to get your combination product to market as soon as possible.
BD PartnerPath™ Program was created to support combination product developers who value speed to market.
Bringing our expertise and practical support to your combination product development can alleviate barriers to your product launch.
BD PartnerPath™ Program offers support in all areas of product development including:

Data
We provide research and development data for delivery systems as part of BD PartnerPath™ Program.*

Drug delivery systems
We help you select the ideal delivery system for your drug, with quick access to small quantities of preconfigured BD products.

Documentation
We deliver documentation to underpin your product development. With our help you can lower the risk of slowing down regulatory approvals.

Technical support
Our experts provide clarity on all types of technical issues related to primary and secondary packing for combination products.
Explore our products
* Data currently available for selected systems
- Source: “Challenges with System Integration" [external study], Franklin Lakes, NJ, USA: GLG 2019 Double blinded qualitative market research with 8 industry experts. Participants worked directly on drug development projects, requiring combination devices (primary and secondary containers) and included the assembly of PFS and/or PFS with autoinjectors.
- Hopkins BP, Miller KJ. Swimming upstream: developing and commercializing diabetes products in a patent protected world. J Diabetes Sci Technol. 2013;7(2):302‐307. Published 2013 Mar 1. doi:10.1177/193229681300700203
- van den Bemt BJF, Gettings L, Domańska B, Bruggraber R, Mountian I, Kristensen LE. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26(1):384‐392. doi:10.1080/10717544.2019.1587043
- Zhaoyang Li & Rachael Easton (2018) Practical considerations in clinical strategy to support the development of injectable drug-device combination products for biologics, mAbs, 10:1, 18-33, DOI: 10.1080/19420862.2017.1392424
- Pourkavoos, N. Unique Risks, Benefits, and Challenges of Developing Drug-Drug Combination Products in a Pharmaceutical Industrial Setting. comb.prod.ther. 2, 2 (2012). https://doi.org/10.1007/s13556-012-0002-2
- ISO11608 requirements for needle injection systems. https://www.iso.org/obp/ui/#iso:std:iso:11608:-1:ed-3:v1:en
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6(8):469‐478.