BD ChloraPrepTM skin antisepsis has been demonstrated to reduce the risk of catheter-related bloodstream infections (CRBSIs)9, Surgical Site Infections (SSIs)10, and contamination of blood cultures11 and donations12 when used for the preparation of the patient’s skin prior to minor and major procedures.
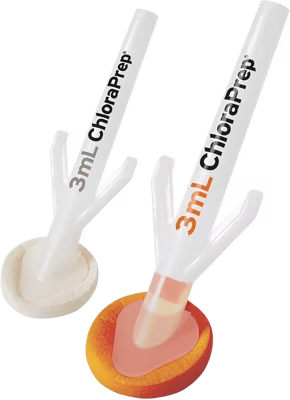
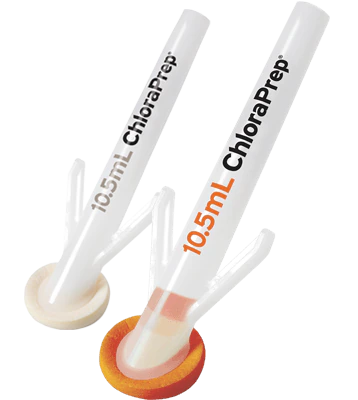
BD ChloraPrepTM applicators deliver our trusted formulation including a sterile 2% w/v chlorhexidine gluconate (CHG) and 70% v/v isopropyl alcohol (IPA) through an easy-to-use design for skin prep. Applicators are available in 1 mL, 1.5 mL FREPP, 3mL, 10.5 mL, and 26 mL sizes.