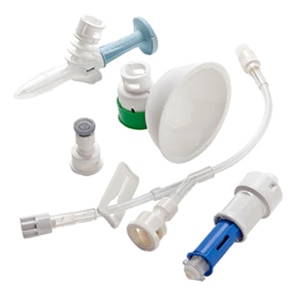
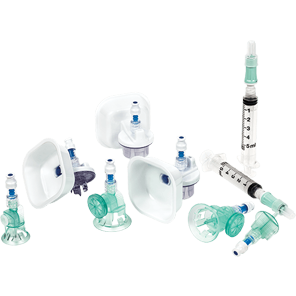
BD PhaSeal™ Optima system
The next-generation Closed-System Drug-Transfer Device (CSTD) from BD that advances hazardous drug protection


- Overview
- EIFU & Resources
Safety. Performance. Ergonomics. Ease of use. We've optimised every component, and the result is a CSTD with design innovations that advance hazardous drug safety. The BD PhaSeal™ Optima system meets the requirements of the NIOSH IPA draft CSTD-test protocol‡, a robust test protocol applicable to barrier-type CSTDs.1,2
Airtight
Every component is designed with no inlets or air exchange for completely airtight hazardous drug transfers.
Demonstrated
The BD PhaSeal™ Optima system prevents microbial ingress for up to 168 hours and 10 penetrations*†
Efficient
The BD PhaSeal™ Optima system minimises residual drug loss in vials compared to other similar system.
User-validated
Feedback from longstanding CSTD users and traditional technique users guided us to optimise and innovate the design.
Safety
- Injector designed to prevent accidental needlesticks
- Self-sealing membranes for leakproof connections
Performance
- Proprietary protector design to maximise drug extraction
- Compatible with ISO-compliant Luer lock syringes and IV sets
Ergonomics
- Power grip and straight motion to connect and disconnect, aligned with NIOSH recommendations to avoid pinch grip3
- Optimised design for clinician comfort
Ease of use
- Intuitive one-step straight-push connection to aid workflow
- Can connect without alignment to help minimise learning curve
BD PhaSeal™ Optima Video for Pharmacy
BD PhaSeal™ Optima for Nursing
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes
‡2015
*Within an ISO Class V environment following aseptic technique.
†The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer’s recommendations and USP compounding guidelines for shelf life and sterility information.
References
- National Institute for Occupational Safety and Health (NIOSH). A vapor containment performance protocol for closed system transfer devices used during pharmacy compounding and administration of hazardous drugs. https://www.cdc.gov/niosh/docket/review/docket288/pdfs/a-vapor-containment-performance-protocol-for-closed-system-transfer-devices.pdf. Accessed May 26, 2020.
- BD-8440 BD PhaSeal Optima Vapor Containment White Paper.
- Easy Ergonomics: A Guide to Selecting Non-Powered Hand Tools. Centers for Disease Control and Prevention website. https://www.cdc.gov/niosh/docs/2004-164/pdfs/2004-164.pdf. Published August 18, 2004. Accessed April 22, 2020.
BD-20816