CapSure™는 강력하고 안정적인 고정을 통해 외과의가 원하는 자신감을 제공합니다.

- 개요
- 제품 및 부속품
- EIFU 및 리소스
- 자주 묻는 질문(FAQ)
탈장 수복을 위한 영구 고정의 전통적인 과제
영구 고정 장치는 장기간의 강력한 회복을 용이하게 하지만 다음과 같은 몇 가지 과제와 관련이 있을 수 있습니다.
- 접착제와 체결구 등 노출된 금속 부분으로 인한 임상 합병증
- 대형 기공 메쉬 고정이 어려워 탈장 수복 결과에 영향을 미칠 수 있음
- 배치 문제나 장치 신뢰성 문제로 인해 시술이 중단될 수 있음
Bard는 영구 고정을 재정의했습니다
커버가 있습니다
- 부드러운 폴리에테르에테르케톤(PEEK) 캡은 노출된 금속 팁을 제거하고 체결구의 유착을 최소화하는 데 도움이 됩니다*
강합니다
- 쿠퍼 인대 및 “P 제품”과 유사한 하위 구조물에 고정합니다
신뢰할 수 있습니다
- 손잡이가 편안하고 트리거 배치가 쉬움
- 일관된 체결구 배치 및 조직 구매 깊이
- 메쉬 기공 크기에 관계 없이 믿을 수 있고 안전한 고정
- “P 제품”에 비해 체결구의 캡 표면이 더 크기 때문에 대형 기공 메쉬로의 고정 개선
*전임상 데이터. 결과는 인간에 대한 성능과 상관관계가 없을 수 있습니다

재정의된 체결구 설계
316L 스테인리스 스틸
- 316L 스테인레스 스틸은 골 나사 및 인공관절을 포함하여 압력을 가하는 생의학 이식에 일반적으로 사용되는 수술용 스테인리스 스틸입니다.
부드러운 PEEK 캡
- 캡은 폴리에테르에테르케톤(PEEK)으로 만들어졌습니다. PEEK는 불활성 유기 열가소성 폴리머로, 고급 생체재료로 간주됩니다
PEEK는 치과용 임플란트, 심장 판막과 스텐트, 관절 보철물을 비롯한 여러 의료용 이식에 사용됩니다
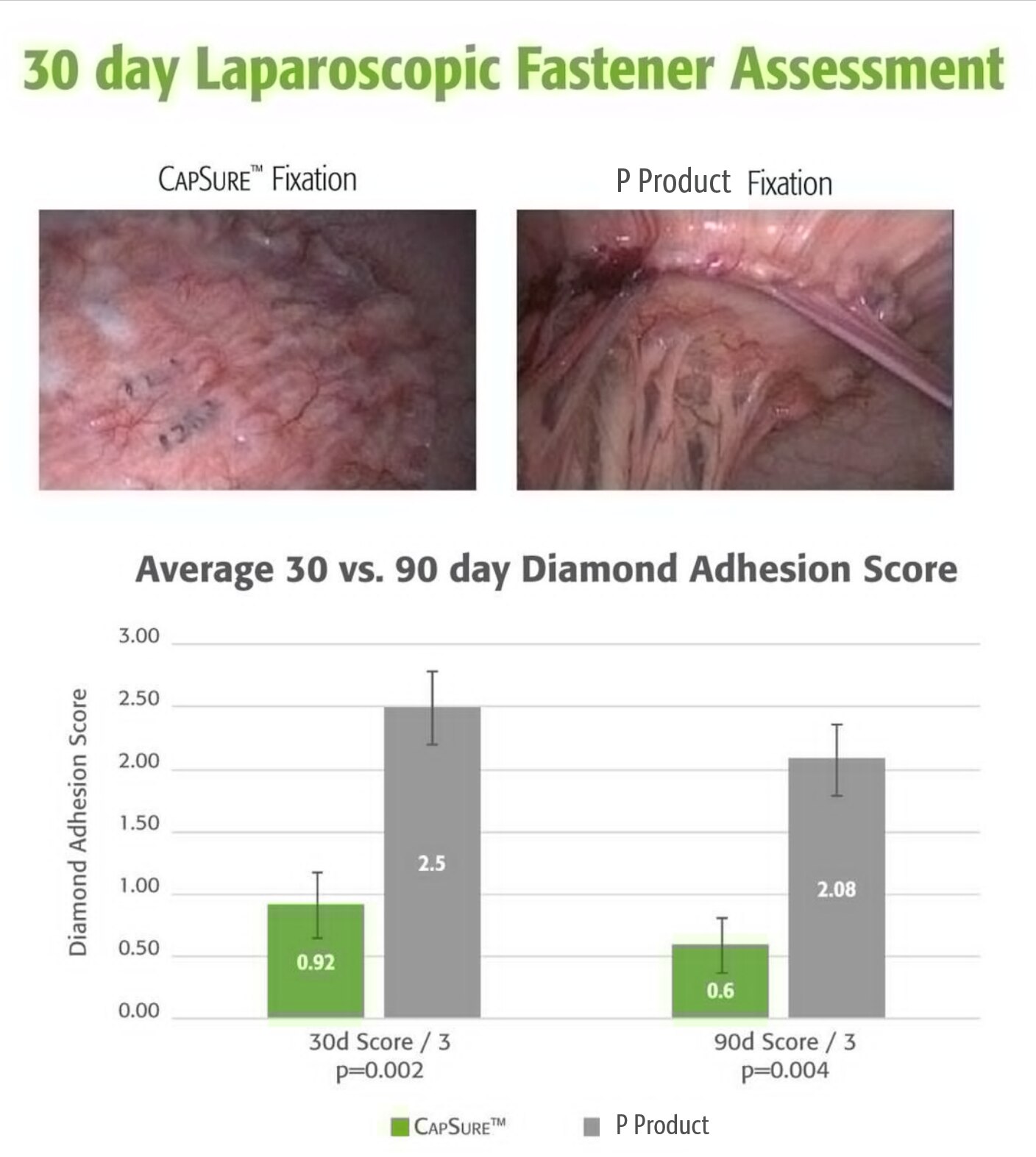
90일 전임상 접착 연구 결과 P 제품 고정 시스템보다 CapSure™ 고정 시스템이 더 강력한 결과를 보였습니다.
Evaluation of a Novel Permanent Capped Helical Coil Fastener in a Porcine Model of Laparoscopic Ventral Hernia Repair
Arnab Majumder, Mojtaba Fayezizadeh, William W. Hope,
Yuri W. Novitsky • Surgical Endoscopy(내시경 복강경 외과학회지) 2016년 4월
- 유의미하게 더 적은 접착 범위
- 제대로 적용된 체결구 퍼센티지 증가
- 메쉬/조직 융합 향상
- 유착 형성을 줄이고 메쉬 고정 및 융합에 도움이 되도록 폴리머 캡으로 내장 메쉬 표면의 노출된 체결구 포인트를 차폐하는 것을 데이터는 권장합니다.
전임상 데이터. 결과는 인간에 대한 성능과 상관관계가 없을 수 있습니다.
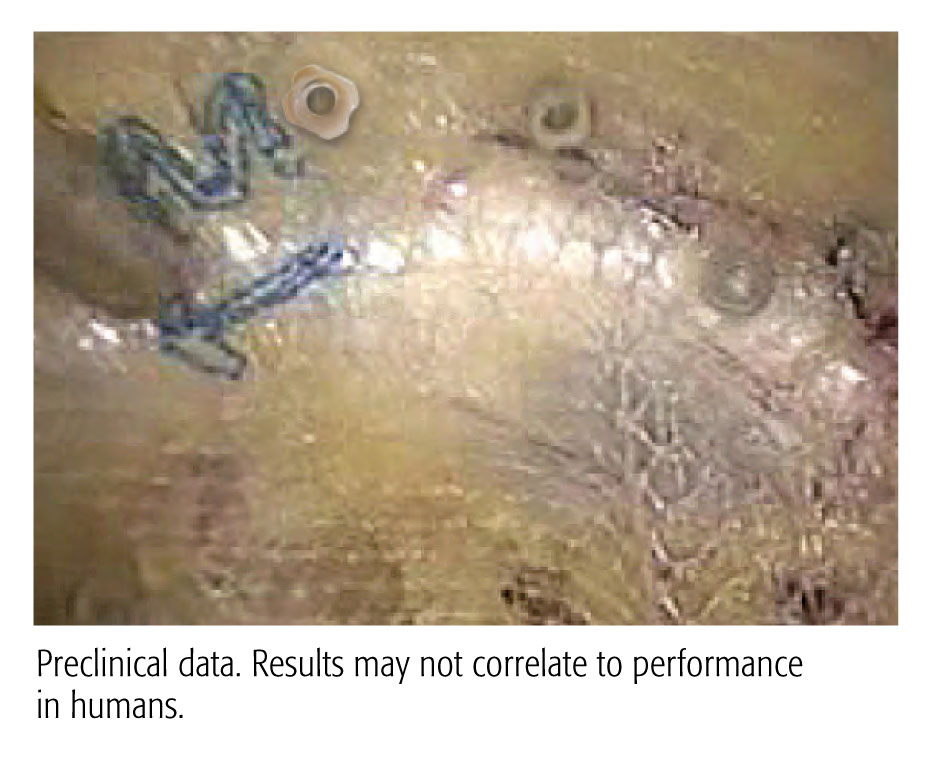
CapSure™ 체결구는 P 제품과 같이 쿠퍼 인대 및 하부 구조를 쉽게 관통합니다
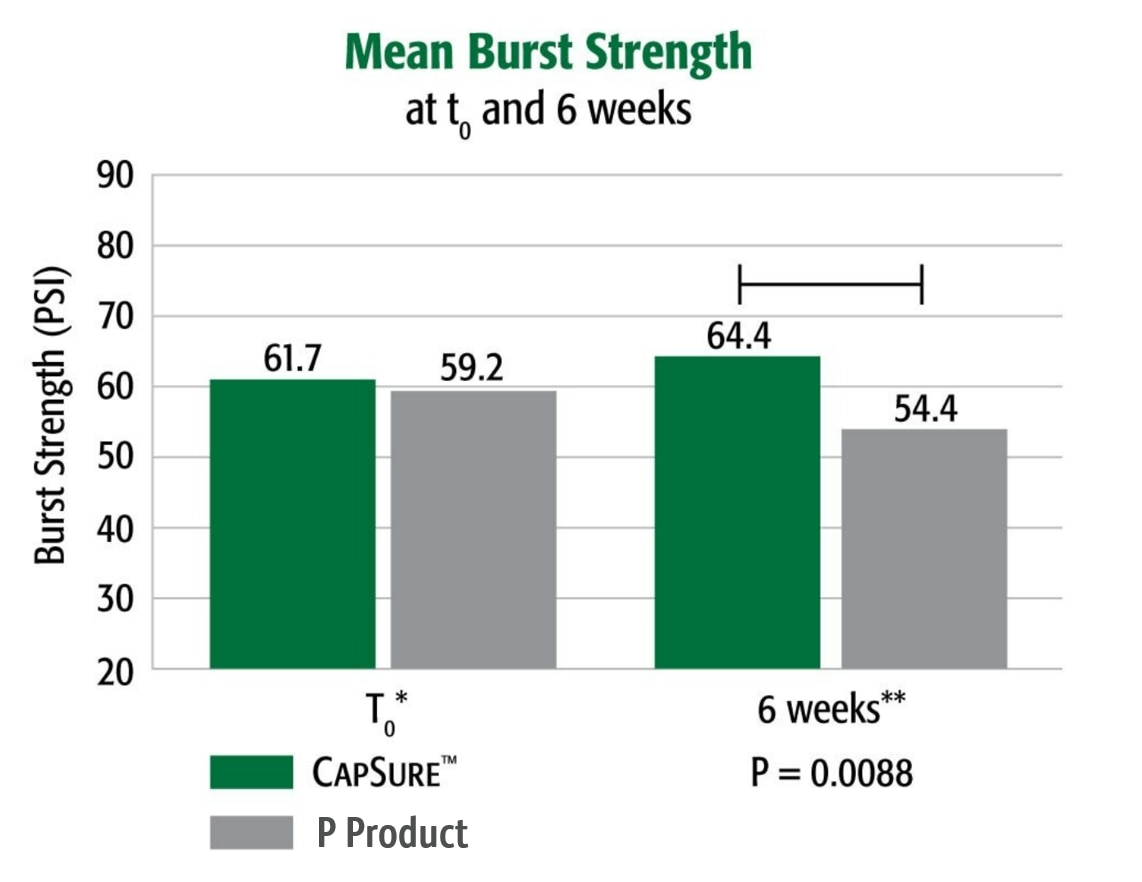
P 제품에 대하여 t0에서는 강도 동일, 시간이 지나며 강도 증가
돼지 모델에서는 CapSure™ 고정 Ventralight™ ST 메쉬의 경우 t0에서 파열 강도가 더 크며(4.3%), 시술 후 6주차에 “P 제품”이 같은 시점(p = .0088)에 Ventralight™ ST 메쉬를 고정한 것보다 피크 파열 강도가 상당히 크다는 것(18.4%)이 파열 강도 시험에서 입증되었습니다.
* 돼지 복벽 조직:
동물 데이터는 사람의 성능과 상관 관계가 없을 수 있습니다
* 돼지 6주 이식 연구:
동물 데이터는 사람의 성능과 상관 관계가 없을 수 있습니다
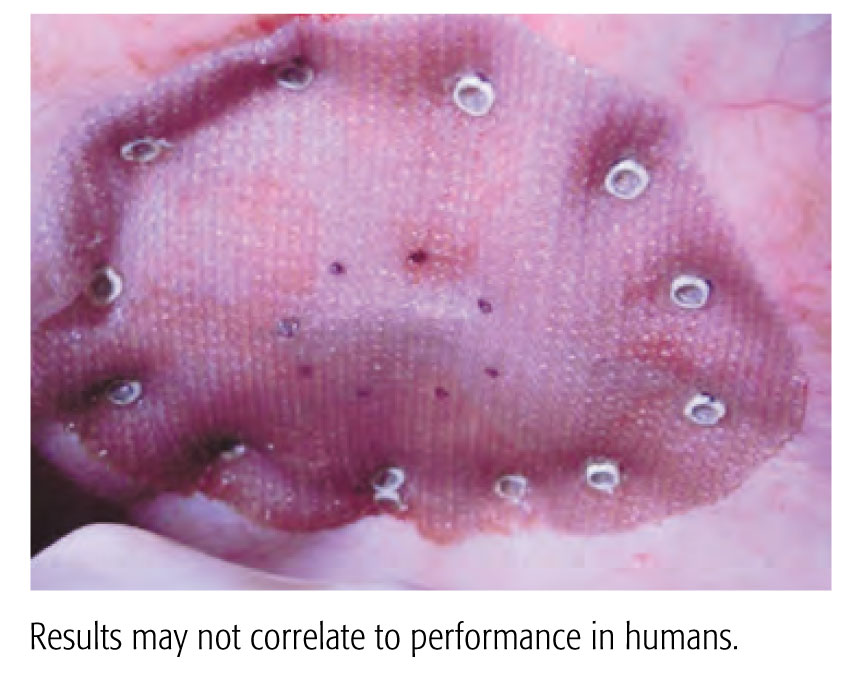
전달 시스템 성능에 대한 신뢰 개선
- 손잡이가 편안하고 트리거 설치가 용이하며 P 제품보다 평균 전달력이 30% 적은 회전 구동 시스템1
- 일관된 체결구 배치 및 조직 구매 깊이 – 전임상 연구에서 P 제품에 비해 더 유리한 체결구 고정 결과를 보임2

CapSure™ 체결구
CapSure™ 체결구는 메쉬 표면 적용범위가 2배이므로 메쉬가 제자리에 고정되어 있고 더 잘 보이도록 고정되어 있습니다. 3DMax™ 경량을 사용하여 벤치 테스트를 한 결과 P 제품에 비해 CapSure™이 대형 기공 메쉬를 유지할 가능성이 15배 더 높다는 것이 입증됨1
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
Precautions
Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
Precautions
Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
Precautions
Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
