TAPP, TEP 및 로봇 TAPP와 같은 복강경 접근법을 위한 임상적으로 입증된 고정이 필요 없는 제품
3DMax™ Mesh 는 서혜부에 대해 신중하고 정밀한 해부학적 연구를 바탕으로 개발되었습니다 . 3 차원의 해부학적 곡선 모양 , 밀봉된 가장자리 및 내측 방향 마커는 기존의 평면 Mesh 보다 위치 지정이 용이하며 배치의 속도와 단순성을 향상시킵니다.1
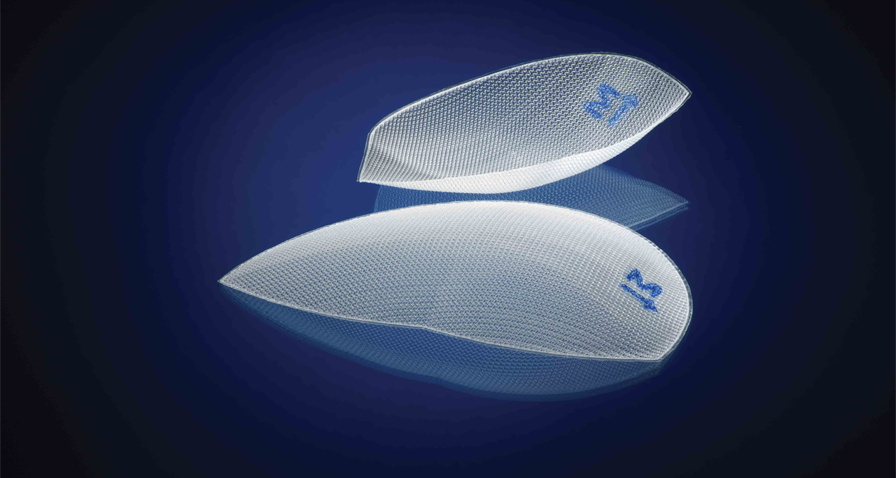

3DMax™ Mesh 는 서혜부에 대해 신중하고 정밀한 해부학적 연구를 바탕으로 개발되었습니다 . 3 차원의 해부학적 곡선 모양 , 밀봉된 가장자리 및 내측 방향 마커는 기존의 평면 Mesh 보다 위치 지정이 용이하며 배치의 속도와 단순성을 향상시킵니다.1
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
3DMax™를 이용한 TEP 복강경 서혜부 탈장 수복
서혜부 해부학적 구조에 맞게 설계된 완벽한 윤곽의 메쉬.
밀봉된 가장자리와 내측 방향 마커는 해부학적으로 정확한 맞춤을 보장하고 기존 평면 메쉬보다 주름이 덜 생기도록 설계되었습니다.
고정할 필요가 없으므로 시간과 비용을 절약할 수 있습니다.
고정 없이 Bard 3DMax™ 메쉬를 시술받은 환자는 평면 메쉬를 고정한 환자보다 수술 직후에 마약성 진통제를 훨씬 적게 사용했습니다.2
1. Bell, Price. “Laparoscopic Inguinal Hernia Repair Using an Anatomically Contoured Three-Dimensional Mesh.” Surgical Endoscopy. 2003:17:1784-1788.
2. Koch et al. “Randomized Prospective Study of Totally Extraperitoneal Inguinal Hernia Repair: Fixation Versus No Fixation of Mesh.” Journal of the Society of Laparoendoscopic Surgeons. 2006:10:457-460.
Indications
Bard 3DMax™ mesh is indicated to reinforce soft tissue where weakness exists, e.g., for repair of hernia and chest wall defects.
Contraindications
Literature reports that there may be a possibility for adhesion formation when Bard 3DMax™ mesh is placed in direct contact with the bowel or viscera. Do not use Bard 3DMax™ mesh in infants and children, whereby future growth will be compromised by use of such material.
Warnings
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the prosthesis. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
Precautions
Do not cut or reshape the Bard 3DMax™mesh as this may affect its effectiveness. If fixation is used, care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used. If sutures are used to secure the mesh in place, nonabsorbable monofilament sutures are recommended.
Adverse Reactions
Possible complications include seromas, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
1. Bell, Price. “Laparoscopic Inguinal Hernia Repair Using an Anatomically Contoured Three-Dimensional Mesh.” Surgical Endoscopy. 2003:17:1784-1788.
2. Koch et al. “Randomized Prospective Study of Totally Extraperitoneal Inguinal Hernia Repair: Fixation Versus No Fixation of Mesh.” Journal of the Society of Laparoendoscopic Surgeons. 2006:10:457-460.
Indications
Bard 3DMax™ mesh is indicated to reinforce soft tissue where weakness exists, e.g., for repair of hernia and chest wall defects.
Contraindications
Literature reports that there may be a possibility for adhesion formation when Bard 3DMax™ mesh is placed in direct contact with the bowel or viscera. Do not use Bard 3DMax™ mesh in infants and children, whereby future growth will be compromised by use of such material.
Warnings
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the prosthesis. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
Precautions
Do not cut or reshape the Bard 3DMax™mesh as this may affect its effectiveness. If fixation is used, care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used. If sutures are used to secure the mesh in place, nonabsorbable monofilament sutures are recommended.
Adverse Reactions
Possible complications include seromas, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
1. Bell, Price. “Laparoscopic Inguinal Hernia Repair Using an Anatomically Contoured Three-Dimensional Mesh.” Surgical Endoscopy. 2003:17:1784-1788.
2. Koch et al. “Randomized Prospective Study of Totally Extraperitoneal Inguinal Hernia Repair: Fixation Versus No Fixation of Mesh.” Journal of the Society of Laparoendoscopic Surgeons. 2006:10:457-460.
Indications
Bard 3DMax™ mesh is indicated to reinforce soft tissue where weakness exists, e.g., for repair of hernia and chest wall defects.
Contraindications
Literature reports that there may be a possibility for adhesion formation when Bard 3DMax™ mesh is placed in direct contact with the bowel or viscera. Do not use Bard 3DMax™ mesh in infants and children, whereby future growth will be compromised by use of such material.
Warnings
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the prosthesis. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
Precautions
Do not cut or reshape the Bard 3DMax™mesh as this may affect its effectiveness. If fixation is used, care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used. If sutures are used to secure the mesh in place, nonabsorbable monofilament sutures are recommended.
Adverse Reactions
Possible complications include seromas, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.