true
지원
Becton, Dickinson and Company
(82.2) 080-340-3800
bd_korea@bd.com
영업팀에 연락해 주셔서 감사합니다!
영업 담당자가 곧 연락을 드립니다.
Address
Becton Dickinson Korea Co., Ltd. 한국 서울특별시 강남구 테헤란로 142 아크플레이스 16층 06236


- 개요
- 제품 및 부속품
- EIFU 및 리소스
TAPP, TEP 및 로봇 TAPP와 같은 복강경 접근법을 위한 가벼운 3D 모양의 메쉬
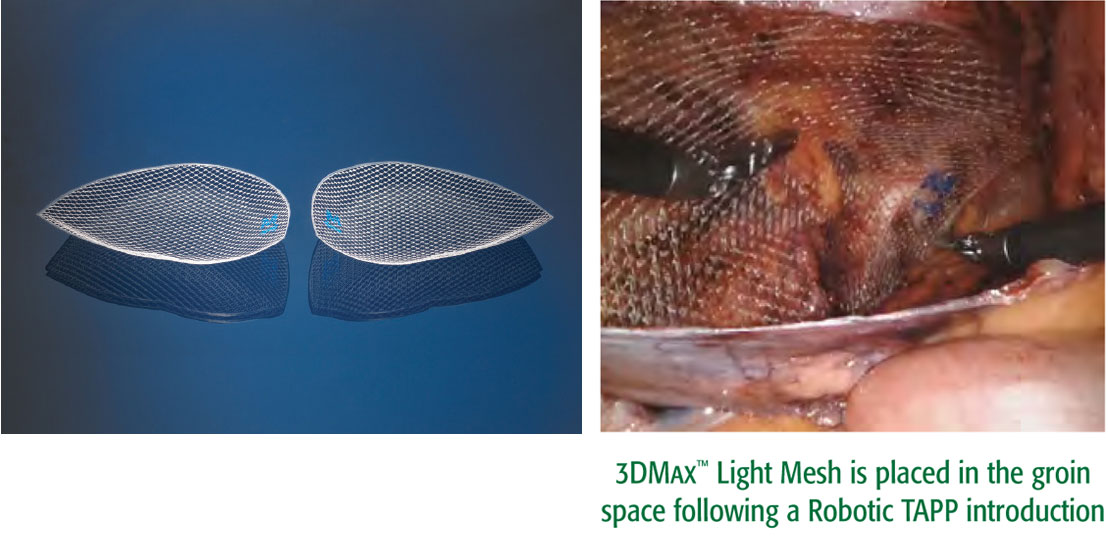
인기 있는 3DMax™ Mesh 의 이 경량버전은 큰 기공 니트가 특징입니다 . 배치가 편리하고 가시성이 뛰어납니다 . 서혜부 해부학적 구조에 맞게 개발되어 로봇 접근법을 포함한 복강경 삽입 후에도 모양이 유지됩니다 .
고유함
- 복강경 외과의가 개발한 3D 모양 .
- 서혜부 해부학적 구조에 맞게
개발되었습니다 . - 윤곽은 평면 메쉬에서 나타날 수
있는 주름을 최소화합니다 . - 설계로 인해 고정의 필요성을 줄일
수 있습니다 .
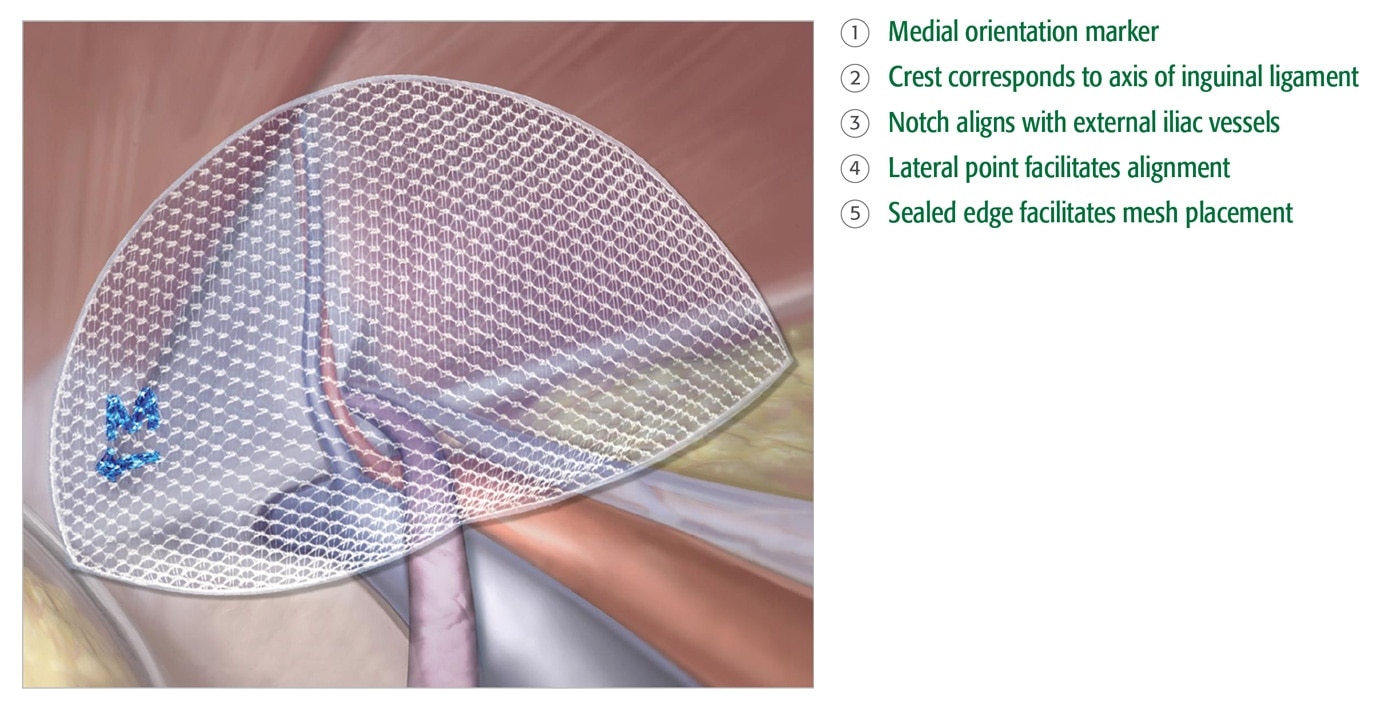
정확함
- 밀봉된 가장자리와 중앙 방향
마커로 정확한 배치와 위치
지정이 가능합니다 . - 내장 메모리가 모양을
유지합니다 . - 3DMax™ Light Mesh 는 3
가지 사이즈로 제공되며 , 왼쪽
및 오른쪽 방향 모두에 사용할
수 있습니다 .
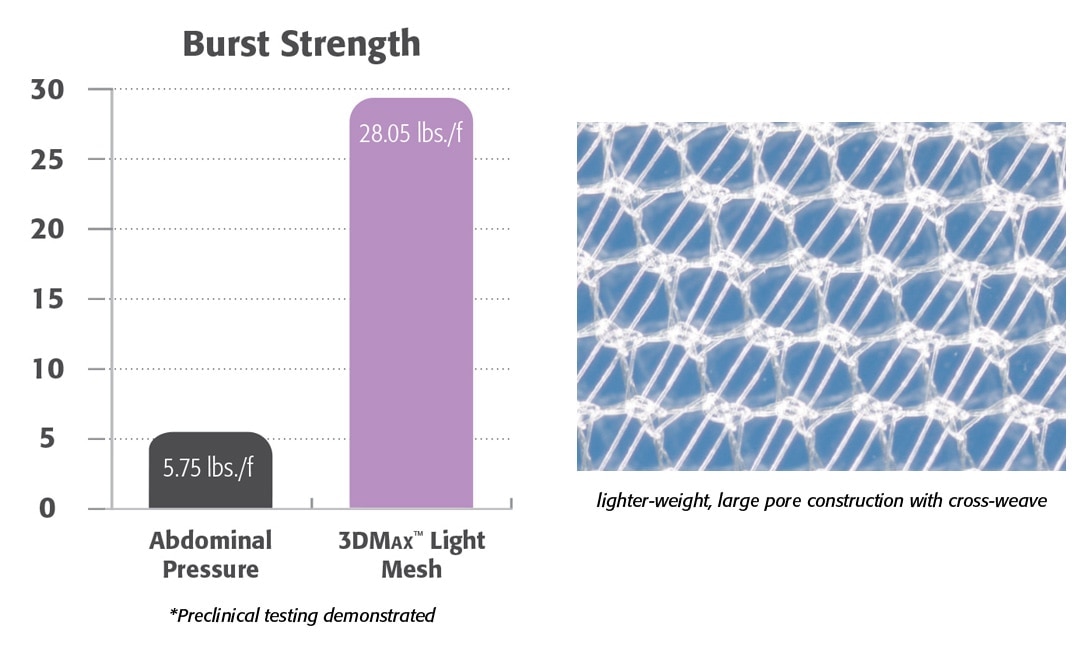
경량
- 경량 모노필라멘트 폴리프로필렌 메쉬.
- 대형 기공 니트는 뛰어난 가시성을 제공합니다.
- 전임상 데이터에 따르면 유연하고 순응적인 복벽이 형성되는 것으로 나타났습니다.1
다양한 복강경 접근법과 호환 가능
- TAPP
- TEP
- 로봇 TAPP
Indications.
The 3DMax™ Light Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal hernias.
Contraindications.
1. Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by use of such materials.
2. The use of this mesh has not been studied in pregnant or breastfeeding women.
3. Literature reports that there may be a possibility for adhesion formation when polypropylene is placed in direct contact with the bowel or viscera.
Warnings.
1. The use of any synthetic mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
3. If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections.
4. To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
5. The mesh is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
6. This mesh had been designed for single use only. Reuse, reprocessing, resterilization, or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness or death of the patient or end user.
7. To avoid injury, careful attention is required if fixating the mesh in the presence of nerves, vessels, or the spermatic cord. Fastener penetration into underlying tissue containing nerves or blood vessels may result in the need for medical/surgical intervention, cause serious injury or permanent impairment to a body structure.
Precautions.
1. Please read all instructions prior to use.
2. Only physicians qualified in appropriate surgical techniques should use this mesh.
3. Do not cut or reshape the 3DMax™ Light Mesh as this may affect its effectiveness.
4. Use an appropriately sized trocar to allow mesh to slide down the trocar with minimal force.
5. If fixation is used, Bard® permanent or absorbable fixation devices or nonabsorbable monofilament sutures are recommended to properly secure the device. If other fixation devices are used, they must be indicated for use in hernia repair.
6. If fixation is used, care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used.
Adverse Reactions.
Possible complications may include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction and recurrence of the hernia or soft tissue defect.
Please consult product package insert for more detailed safety information and instructions for use.
Please consult package insert for more detailed safety information and instructions for use.
true
Indications.
The 3DMax™ Light Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal hernias.
Contraindications.
1. Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by use of such materials.
2. The use of this mesh has not been studied in pregnant or breastfeeding women.
3. Literature reports that there may be a possibility for adhesion formation when polypropylene is placed in direct contact with the bowel or viscera.
Warnings.
1. The use of any synthetic mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
3. If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections.
4. To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
5. The mesh is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
6. This mesh had been designed for single use only. Reuse, reprocessing, resterilization, or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness or death of the patient or end user.
7. To avoid injury, careful attention is required if fixating the mesh in the presence of nerves, vessels, or the spermatic cord. Fastener penetration into underlying tissue containing nerves or blood vessels may result in the need for medical/surgical intervention, cause serious injury or permanent impairment to a body structure.
Precautions.
1. Please read all instructions prior to use.
2. Only physicians qualified in appropriate surgical techniques should use this mesh.
3. Do not cut or reshape the 3DMax™ Light Mesh as this may affect its effectiveness.
4. Use an appropriately sized trocar to allow mesh to slide down the trocar with minimal force.
5. If fixation is used, Bard® permanent or absorbable fixation devices or nonabsorbable monofilament sutures are recommended to properly secure the device. If other fixation devices are used, they must be indicated for use in hernia repair.
6. If fixation is used, care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used.
Adverse Reactions.
Possible complications may include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction and recurrence of the hernia or soft tissue defect.
Please consult product package insert for more detailed safety information and instructions for use.
Please consult package insert for more detailed safety information and instructions for use.
true
true
true
Indications.
The 3DMax™ Light Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal hernias.
Contraindications.
1. Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by use of such materials.
2. The use of this mesh has not been studied in pregnant or breastfeeding women.
3. Literature reports that there may be a possibility for adhesion formation when polypropylene is placed in direct contact with the bowel or viscera.
Warnings.
1. The use of any synthetic mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
3. If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections.
4. To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
5. The mesh is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
6. This mesh had been designed for single use only. Reuse, reprocessing, resterilization, or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness or death of the patient or end user.
7. To avoid injury, careful attention is required if fixating the mesh in the presence of nerves, vessels, or the spermatic cord. Fastener penetration into underlying tissue containing nerves or blood vessels may result in the need for medical/surgical intervention, cause serious injury or permanent impairment to a body structure.
Precautions.
1. Please read all instructions prior to use.
2. Only physicians qualified in appropriate surgical techniques should use this mesh.
3. Do not cut or reshape the 3DMax™ Light Mesh as this may affect its effectiveness.
4. Use an appropriately sized trocar to allow mesh to slide down the trocar with minimal force.
5. If fixation is used, Bard® permanent or absorbable fixation devices or nonabsorbable monofilament sutures are recommended to properly secure the device. If other fixation devices are used, they must be indicated for use in hernia repair.
6. If fixation is used, care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used.
Adverse Reactions.
Possible complications may include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction and recurrence of the hernia or soft tissue defect.
Please consult product package insert for more detailed safety information and instructions for use.
Please consult package insert for more detailed safety information and instructions for use.
true
true
