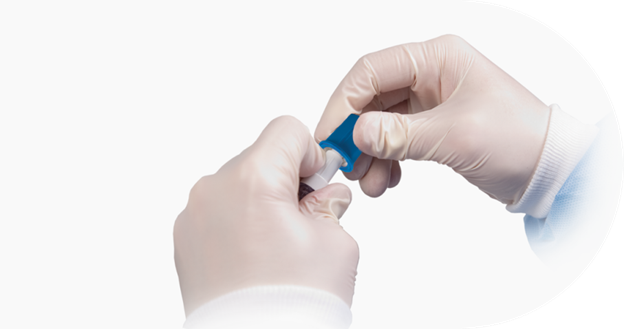
The BD Site-Scrub™ IPA Device is an active disinfecting device intended for use on injection ports and female luer hubs. It is designed with patented “foam fingers” containing 70% isopropyl alcohol (IPA) solution which provide active friction scrubbing to surfaces of the luer.
BD® Site-Scrub™ IPA Device
A versatile disinfecting device intended for use on injection ports and female luer hubs

- Overview
- Products & Accessories
- EIFU & Resources

Scrub and go
Just 8 simple twists back and forth to scrub over 10 seconds, followed by a 5-second dry to disinfect effectively and easily.1

Patented and aligned to CDC guidelines
Patented 70% IPA-infused “foam finger” design, intended for use on injection ports and female luer hubs as a disinfecting cleaner, to scrub the inside and outside surfaces of the luer. This design aligns with CDC guidelines.
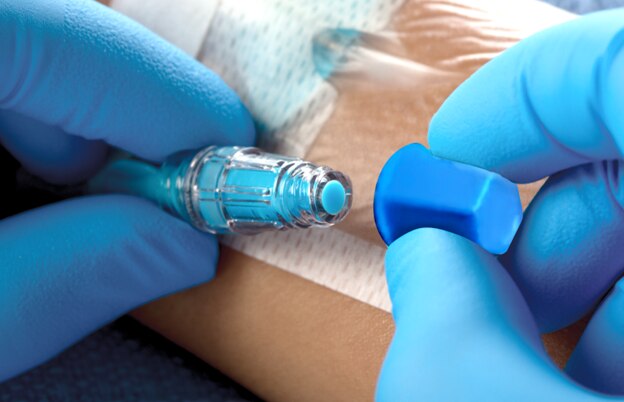
≥ 4-log10 reduction (≥ 99.99%)
In vitro antimicrobial efficacy results show the BD Site-Scrub™ IPA Device provides a 4-log10 (≥ 99.99%) reduction in 6 microorganisms: S. aureus (MRSA), S. epidermidis (MRSE), Pseudomonas aeruginosa, E. coli, Candida parapsilosis and Candida albicans.*1
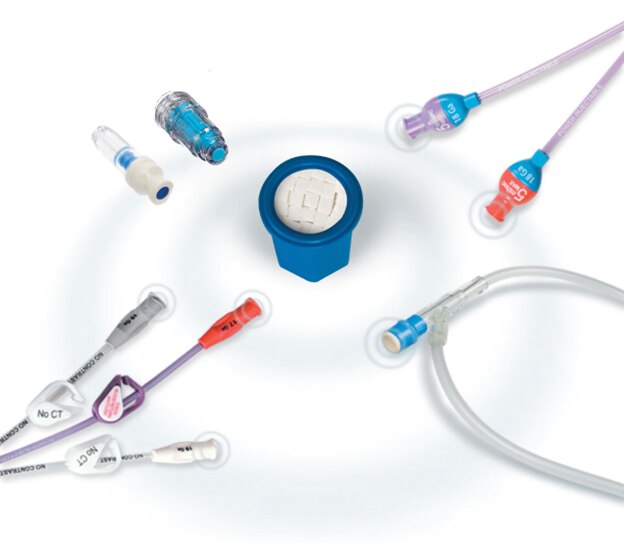
Versatile use
The BD Site-Scrub™ IPA Device is intended to disinfect needleless access devices such as female luers, hubs, injection ports, y-sites and stopcocks.

Explore the all-in-one BD PosiFlush™ SafeScrub Prefilled Saline Flush Syringe with an active disinfection unit built into the tip cap of the syringe

Find out why disinfecting caps like BD PureHub™ Disinfecting Caps should be an integral part of your disinfecting protocol
*In vitro testing may not be indicative of actual clinical performance.
Indications for use: The BD Site-Scrub™ IPA Device is intended for use on injection ports and female luer hubs as a disinfecting cleaner.
Warning: Do not use on devices which access the nervous system. Please consult product labels and inserts for any indications, contraindications, hazards, warnings, cautions and instructions for use. Do not leave the BD Site-Scrub™ IPA Device on an injection port or female luer hub after disinfecting.
References:
1. BD Study: C-1608 BD Site-Scrub™ 4-log reduction. 2020.
2. CDC. Guidelines for the Prevention of Intravascular Catheter-Related Infections (2011) Summary of Recommendations. CDC website. https://www.cdc.gov/infectioncontrol/guidelines/bsi/recommendations.html
BD-131280 (03/25)

