INDICATIONS FOR USE:
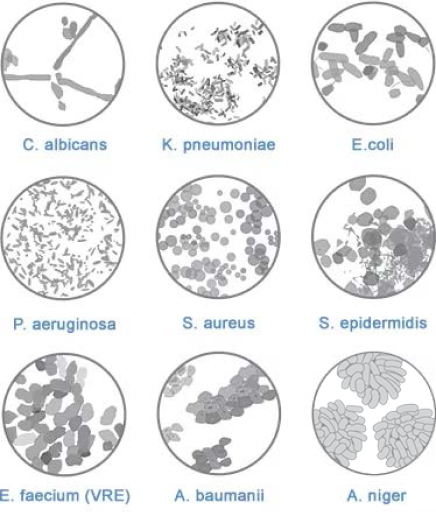
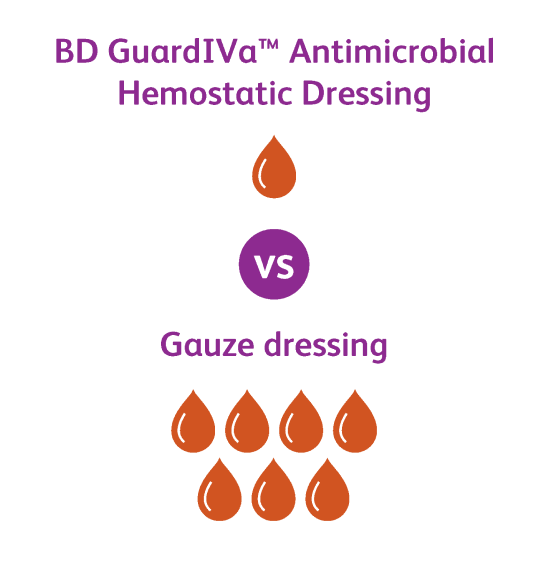
The GuardIVa™ Antimicrobial Hemostatic IV Dressing is intended for use as a hydrophilic wound dressing to absorb exudate, cover and protect catheter sites. Common applications include IV catheters, other intravenous catheters and percutaneous devices. It is also indicated for control of surface bleeding from percutaneous catheters and vascular access sites.
WARNINGS:
Do not use the GuardIVa™ Antimicrobial Hemostatic IV Dressing on patients with a known sensitivity to chlorhexidine gluconate. The use of chlorhexidine gluconate containing products has been reported to cause irritation, sensitization, and generalized allergic reactions. If any such reactions occur, discontinue use of the dressing immediately, and if severe, contact a physician. For external use only. Do not allow this product to contact ears, eyes, mouth or mucous membranes.
PRECAUTION:
GuardIVa™ Antimicrobial Hemostatic IV Dressing is not intended to treat infection. Please consult product labels, IFU, and package inserts for any indications, contraindications, hazards, warnings, cautions, and instructions for use.