-
BD Viper™ 2 Gallon Liquid Waste Bottle
SKU/REF 441351
-
BD Viper™ LT PCR Tube/Carrier Accessory Kit
SKU/REF 442957
-
BD Viper™ LT SDA Accessory Kit
SKU/REF 442958
-
BD Viper™ LT Seismic Table
SKU/REF 441983
-
BD Viper™ LT System
SKU/REF 442839
-
BD Viper™ LT System Instrument Manual
SKU/REF 443506
-
BD Viper™ LT Waste Bag
SKU/REF 442968
-
BD Viper™ Microwell Package 8 EA
SKU/REF 440752
-
BD Viper™ One Gallon Liquid Waste Bottle
SKU/REF 441072
-
BD Viper™ SDA Extraction Reagent Trough
SKU/REF 441994
-
HPV CLICK CARD 1000
SKU/REF 443747
-
Swab Diluent for the BD ProbeTec™ Qx Amplified DNA Assays
SKU/REF 441361
BD Viper™ LT system
Be her advocate for more precise HPV testing with extended genotyping to identify HPV types beyond 16, 18 and 45


- Overview
- Products & Accessories
- EIFU & Resources
The BD Viper™ LT system offers fully automated molecular testing for the BD Onclarity™ HPV assay – the only FDA-approved assay with extended genotyping – out of BD SurePath™ Liquid-based Pap Test and the Hologic ThinPrep® Pap Test.

- Extended genotyping
Can individually identify HPV 31, which poses a higher risk for cervical precancer as compared to HPV 18
- Risk factor traceability
Can track genotype-specific high-risk HPV persistence, the most important determinant of cervical cancer risk in women who test HPV-positive, regardless of HPV genotype.

Efficiency
- Compact, integrated, self-contained table top system
- Room temperature reagent storage
- Sample to results within a single system
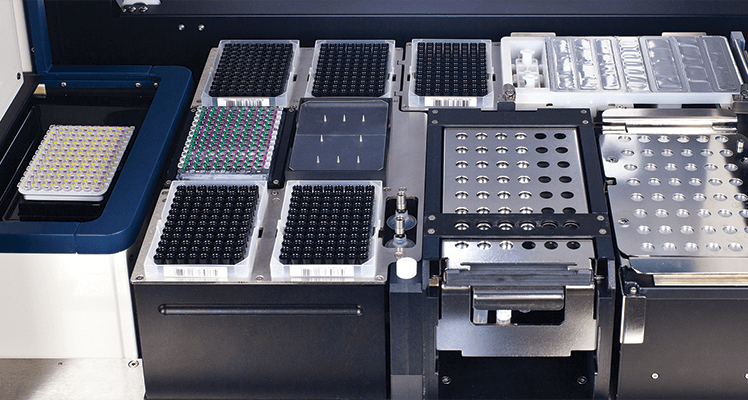
Flexibility
- Supports bi-directional LIS integration
- Middleware solution through BD Totalys™ Datalink
- Remote support through BD Assurity Linc™ to help maximize system uptime
Performance
- Standardized ready-to-use reagents
- <15 minutes hands-on time for run setup
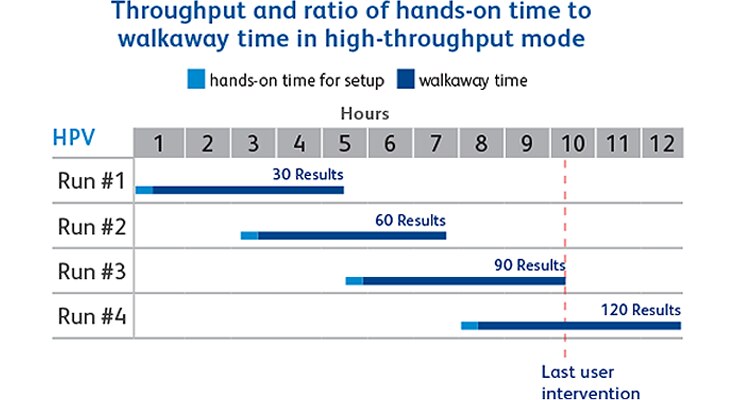
- Up to 30 sample results in 4.5 hours
- 120 results per day. Last user intervention at 9.5 hour in high-throughput mode
- Perform interleaved runs, improving throughput by staggering batches
Comprehensive results
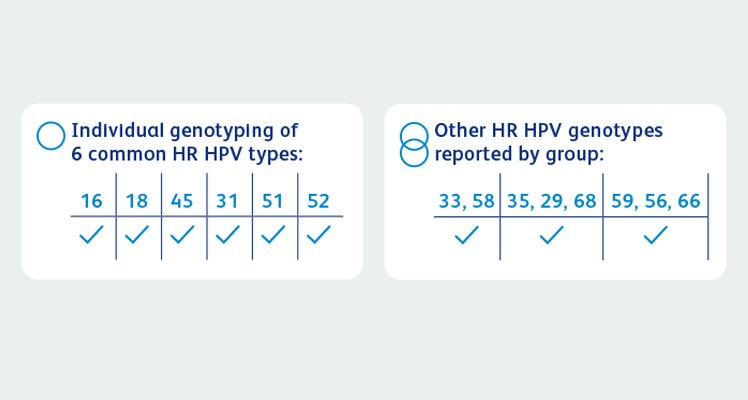
- BD Onclarity™ HPV Assay with extended genotyping is a multiplex real-time PCR assay for the qualitative detection of 14 high-risk HPV types
- On-demand genotyping for individual results of 16, 18, 45, 31, 51 and 52 with pooled results of 33/58, 35/39/68 and 56/59/66
- E6/E7 DNA target
- Utilizes human beta globin as an internal control

The BD SurePath™ Collection Vial offers simple standardized cell collection with positive sample identification and the convenience of being a single source for cytology and molecular testing.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.


