
- Overview
- Products & Accessories
- EIFU & Resources
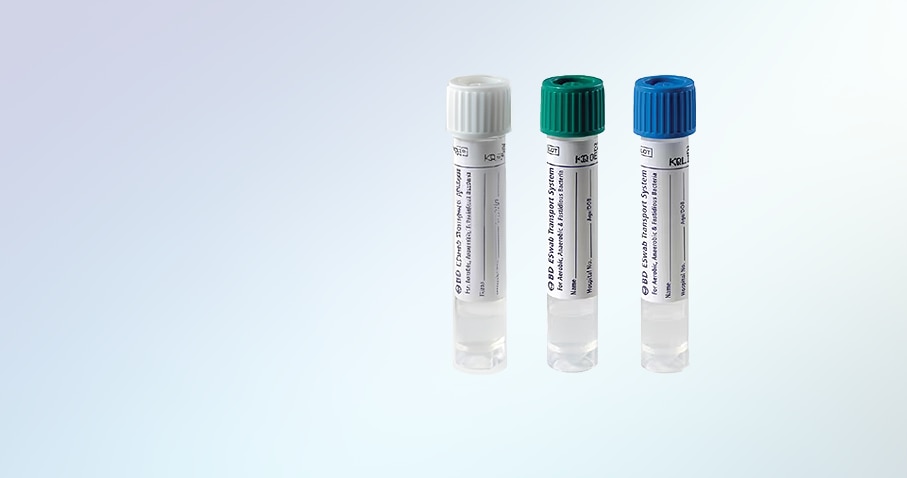
The BD ESwab® collection and transport system is used to collect clinical specimens containing aerobic, anaerobic and fastidious bacteria from the collection site, and transport them to the testing laboratory. In the lab, BD ESwab® specimens are processed using standard clinical lab procedure for bacterial culture. The ESwab® combines a flocked swab with 1mL of Liquid Amies in a plastic screw cap tube. The innovative system elutes over 90% of patient specimen into liquid medium.
-
Modified Liquid Amies
The BD ESwab® system is 1mL of modified Liquid Amies medium packaged with a nylon flocked swab.
-
Automation Ready
The liquid-based microbiology system is easily processed on automated specimen processors and liquid handling pipetting systems, minimizing manual handling.
-
Multiple Swab Options
The system is available in three flocked swab formats: regular, minitip and flexible minitip.
-
Flexibility
The system offers flexibility in transport temperature ranges: in room or refrigerated temperature for up to 48 hours.
-
Swab Differentiation
Color-coded caps offer easy flocked swab differentiation: White = regular, Green = minitip, Blue = flexible minitip.
-
Ease of use
The system is easy to use; just collect, snap and elute.
-
Viability
The system sustains the viability of aerobic, anaerobic and fastidious bacteria in a single formulation.
-
Liquid-based Samples
The BD ESwab® system provides 1mL of samples suspension for Gram stain, multiple culture plates, rapid antigen tests and molecular tests.
Multiple investigations can be performed from the same BD ESwab® sample:
- Multiple culture plates
- Molecular testing*
- Rapid antigen tests*
- Gram stains
For patients, this means fewer samples need to be collected offering a more comfortable experience.
For laboratories, BD ESwab® provides a broad range of testing applications replacing the traditional practices requiring multiple swabs with just one ESwab® thus eliminating costs associated with stocking numerous swab types.
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
Multipurpose BD ESwab® offers value to laboratories and point of care providers
- Improved pathogen recovery for traditional bacteriology culture versus traditional rayon swabs
- Liquid based system easily processed on automated specimen processors and liquid handling pipetting systems, minimizing manual handling
- Homogeneous sample for more consistent and precise Gram stains
- Can be used for Molecular and Rapid Antigen testing on an increasing number of manufacturer’s platform
BD ESwab® has been tested and validated in full compliance with CLSI M40-A2: Quality Control of Microbiological Transport System Standard. The FDA cleared collection and transport system is suitable for aerobic, anaerobic and fastidious bacteria maintaining viability for up to 48 hours at room or refrigerator temperature (Neisseria gonorrhoeae survival at 24 hours per CLSI standard).
Making the change to liquid-based collection and transport is easy
BD’s change management team is available to guide you during training and validation in support of new product implementation.
ESwab is a registered trademark of COPAN ITALIA S.P.A.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.




