

- Overview
- Assays
- Services
Discover the life inside the BD COR™ System
Delivering efficiency, flexibility, and performance to meet your laboratories' evolving molecular diagnostic needs. The BD COR™ System uses multiplex PCR assay design and requires less than 1 hour of daily hands-on time for the full BD COR™ system workflow and only 2 interactions.
Efficiency
- Fully integrated automation from specimen to result
- Minimal interaction required to maintain a high level of system throughput
- Up to 330 sample results on the BD COR™ GX instrument and 1248 sample results on the BD COR™ MX dual instrument configuration when consumables are fully reloaded to get 2 shifts of results with 1 shift of work
Performance
- Modular and scalable to deliver needed throughput and overall system output
- Remote monitoring with internal cameras and built-in redundancy to maximize uptime
- Multiplex PCR assay design supports accurate diagnostics
Flexibility
- Distinct instrument configurations based on your laboratory’s workflow needs through robust laboratory assessments
- Random and continuous access that requires less than 1 hour of daily hands-on time for the full BD COR™ workflow with only 2 interactions
- Ability to use different collection types depending on the assay being run – BD SurePath™ Liquid-based Pap Test, Hologic ThinPrep® Pap Test, BD Molecular Swab Collection Kit, and BD Molecular Urine Transport Kit
BD COR™ System Products
- Enhances laboratory operations and patient management with advanced molecular diagnostics capabilities
- Delivers efficiency, flexibility and performance to meet laboratories evolving molecular diagnostic needs.
- Provides complete chain of custody and is the only high throughput fully integrated pre-analytical and analytical system on the market.

The GX Instrument is an HPV analytical instrument that automates the BD Onclarity™ HPV Assay with extended genotyping, requiring minimum interactions per shift at maximum throughput.
Minimal time at the instrument
- Less than 15 minutes to fully load instrument
- Remote system management and alert capabilities
- High capacity for reagents, consumables, samples and system waste
Extended walk-away time and maximum throughput
- Approximately 7 hours of walk-away time at maximum throughput
- Up to 180 HPV samples with extended genotyping without user intervention
- Ready-to-use barcoded reagents stored at room temperature

The PX Instrument* automates preanalytical steps to simplify and standardize workflows, reducing staff interaction and maximizing walk-away time.
Complete pre-analytic automation
- Automatically converts, vortexes, uncaps, aliquots, and recaps LBC vials
- Preforms all necessary prewarming and cooling steps
Integrated
- Automatically delivers sample to analytical instruments
- Coordinates sample batching to ensure maximum throughput without user involvement
- Barcode-drive chain of custody for full traceability and follow-up testing
High storage capacity
- 480 BD SurePath™ Liquid-based Pap Test Vials
- 2,140 BD Collection Devices for IVD Assays
- Reduces hands-on-time, which may reduce the potential for human error

The MX instrument is a molecular diagnostics analytical instrument for integrated automation of an expanding list of high-demand, essential assays in women’s health and infectious disease testing.
Maximum throughput
- Dual BD COR™ MX configuration can process 1,872 sample results in less than 24 hours
- One BD COR™ MX configuration has capacity for 17 different assays and up to 1,000 sample results with one 8-hour shift of work
- Approximately 7 hours of unimpeded system processing
Minimal system interaction and assay variety
- Assay reagents do not require reconstitution and are stored at room temperature
- Less than 15 minutes to fully load consumables
- Essential assays for sexually transmitted infections, women’s health, and other infectious diseases
Voltage | 100-240 VAC |
Frequency | 50-60 Hz |
Current | Single-phase circuit 15 A |
Temperature | 18°C – 27°C / 64.4°F – 80.6°F |
Humidity | 20% to 80% |
Altitude | Up to 2,000 m (6,562 ft) |
Noise metrics | < 67 dB |
Specimen collection type |
|
Reagent/consumable capacity |
|
Waste capacity |
|
*Capacity and throughput may vary based on specimen types.
BD COR™ System Assays
The BD COR™ System revolutionizes women's health and infectious disease diagnostics, addressing critical clinical gaps with cutting-edge automation. The Women's Health assays on the BD COR™ System represent years of meticulous research and development, showcasing exceptional clinical precision and reliability. Click here Take a deep dive to learn more about BD Women's Health Assays and how we are partnering with laboratories and providers to shape the future of Women’s Health.
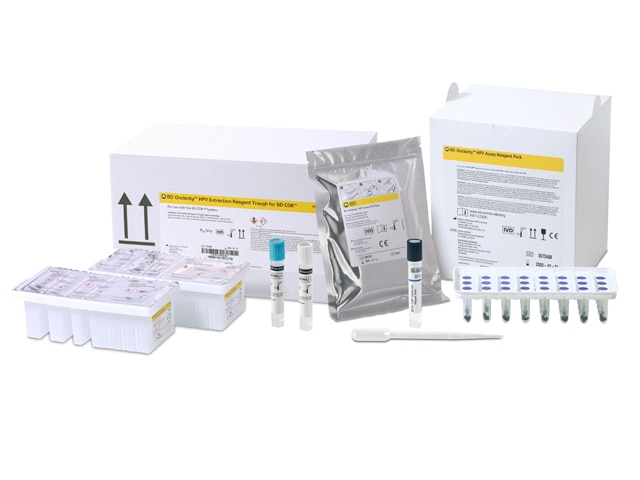
BD Onclarity™ HPV Assay for BD COR™ System
The BD Onclarity™ HPV Assay was the first FDA-approved HPV assay offering extended genotyping. It can be run using both BD SurePath™ Liquid-based Pap Test or Hologic ThinPrep® Pap Test. This innovative technology propels your laboratory to the cutting edge in cervical cancer screening, empowering you to deliver comprehensive and actionable information to clinicians for more precise patient care. Uncover more about how the BD Onclarity™ HPV assay can give you the most comprehensive HPV results on the market today that individually identifies 6 genotypes
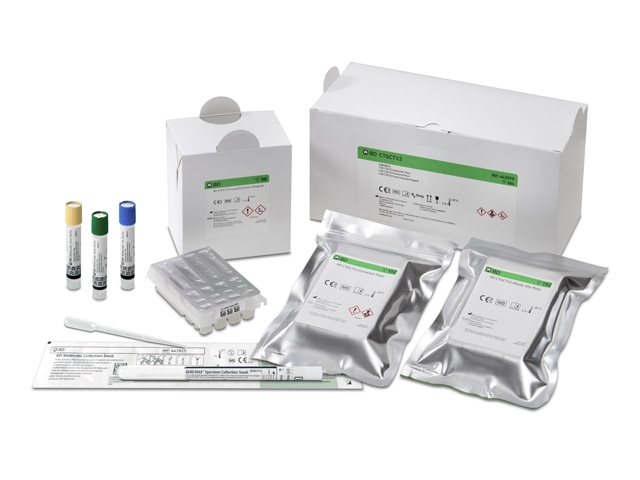
The BD CTGCTV2 assay is seamlessly integrated with BD Molecular and BD Urine specimen collection and transport devices for male and female urine, vaginal, endocervical swabs, and liquid-based cytology specimens on the Hologic ThinPrep® Pap Test. The assay is also indicated for use with asymptomatic and symptomatic individuals to aid in the diagnosis of chlamydial urogenital disease, gonococcal urogenital disease and/or trichomoniasis. Explore the possibilities of testing trichomoniasis at the same time as GT/GC on the BD CTGCTV2 assay.

BD Vaginal Panel for
BD COR™ System
The BD Vaginal Panel is a comprehensive diagnostic test that directly detects the three most common infectious causes of vaginitis – bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and Trichomonas vaginalis (TV) – in one test, using a single swab. This panel is specifically designed to help eliminate ambiguity in test results by providing clear positive or negative outcomes for each condition. Additionally, it delivers separate outcomes for Candida glabrata and C. krusei, two Candida species known for their resistance to conventional antibiotics. Find out more about how BD Vaginal Panel assay is shaping the future of Women's Health.

BD Vaginal Panel and BD CTGCTV2 for BD COR™ System
BD Vaginal Panel and BD CT/GC/TV2 for BD COR™ System can use the same sample to test for vaginitis and the 3 most common non-viral STIs in symptomatic women. 1 Sample, 5 Conditions, 7 Results. Explore more about how BD Vaginal Panel and BD CTGCTV2 can be used together to help shape the future of Women's Health.
BD COR™ System Service Offering
BD Technical Services is your partner for performance. BD Service teams enable you to better serve your customers and patients. You can count on our deep knowledge and experience to help ensure your BD COR™ System delivers expected, timely results.
BD Life Sciences Technical Services
BD Service teams enable you to better serve your customers, patients and research partners. You can count on our deep knowledge and experience to help ensure your instruments deliver expected, timely results.
BD Life Sciences RSS Remote Connectivity
Remote Support Services (RSS) is the BD enterprise-class solution offering remote management and support of BD products deployed at your clinical and research laboratory.

