- Overview
- Clinical Evidence

Simplify and enhance experiences from procurement to insertion with an all-in-one closed IV catheter system1,2
Over 5,000 satisfied customers in the United States
Overall clinician satisfaction has been shown to be significantly higher with Nexiva™ Closed IV Catheter System.*,2
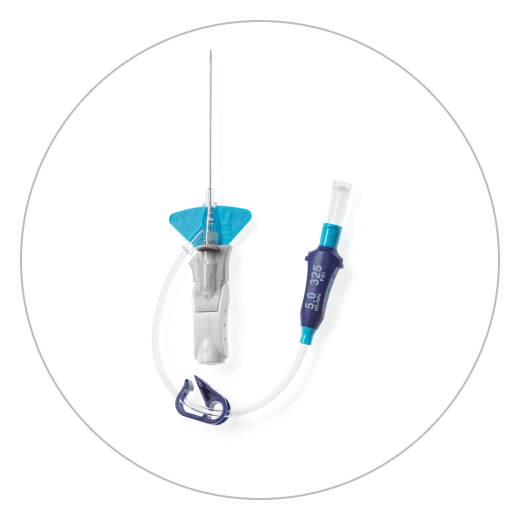
Nexiva™ Catheter Product Family is a comprehensive portfolio of closed, integrated peripheral IV catheters (PIVCs). Each product is built on the Nexiva™ platform and includes a catheter, catheter hub, extension tube and stabilization platform–all in one system.
*compared to a non-integrated PIVC in a clinical study
Backed by 15 years of evidence
Nexiva™ Catheter System has been shown to deliver higher clinician satisfaction, improved clinician safety and a reduction in overall complication rates versus non-integrated, open systems.†,1–3
Count on a system that has been on the market for over 15 years and is backed by evidence in peer-reviewed journals from as early as 20094 to as recently as 2024.5
†with a PTFE catheter
Address common challenges in CT
Nexiva Diffusics™ Closed IV Catheter System is built on the Nexiva™ platform and is differentiated by a fenestrated tip specifically designed for the delivery of contrast media via power injection. It addresses common challenges in CT, providing sufficient flow rates and catheter stability in the vein during power injection procedures.
Enable needle-free blood collection
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Nexiva NearPort™ IV Access is also built on the Nexiva™ platform. It is specifically designed for needle-free blood collection using the compatible PIVO™ Pro Needle-Free Blood Collection Device.
Find the system that meets the needs of you and your patients
Explore the comprehensive Nexiva™ Catheter Product Family portfolio. Click to learn more about each configuration.
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Rickard CM, Larsen E, Walker RM, et al. Integrated versus nonintegrated peripheral intravenous catheter in hospitalized adults (OPTIMUM): A randomized controlled trial. J Hosp Med. 2022;1-12.
- Paterson R, Larsen E, Cooke M, et al. Integrated versus non-integrated peripheral intravenous catheters: a cross-sectional survey of nurse experiences. Br J of Nurs. 2023, Vol 32.
- McNeil E, Hines N, Phariss R. A clinical trial of a new all-in-one peripheral-short catheter. JAVA. 2009;14(1):46-51.
- Gidaro A, Quici M, Giustivi D, et al. Integrated short peripheral intravenous cannulas and risk of catheter failure: a systematic meta-analysis. J Vasc Access. 2024.
BD-117237 (05/24)
Clinical evidence
Nexiva™ Catheter Product Family is built on the Nexiva™ platform.
Nexiva™ Closed IV Catheter System is backed by evidence in peer-reviewed journals from as early as 20091 to as recently as 2024.2
BD Nexiva Diffusics™ Closed IV Catheter System is also backed by peer-reviewed journals and infusion guidelines. Infusion Therapy Standards of Practice state to consider a fenestrated catheter for a contrast-based radiographic study and in coronary CTA.3 Nexiva Diffusics™ Catheter System is the only peripheral IV catheter with a diffusion catheter tip specifically designed for power injection.
Closed IV Catheter System | Comparative
| Data gathered | Year published | Study type | Journal | Authors |
| Nexiva™ | Various | Catheter failure, dislodgement, occlusion, phlebitis | 2024 | Systematic review/meta analysis2 | Journal of Vascular Access | Gidaro A, Quici M, Giustivi D, et al. |
| Nexiva™ | BD Insyte Autoguard™ | Complications, dislodgement, dwell time, infiltration, occlusion, phlebitis | 2023 | RCT4 | European Journal of Oncology Nursing | Gerceker GO, Yildirim BG, Onal A, et al. |
| Nexiva™ | B. Braun Introcan Safety® BD Insyte Autoguard™ | Catheter-associated bloodstream infection, clinician satisfaction, cost savings/utilization reduction, dwell time, dislodgement/leaking, infiltration/extravasation, local infection, insertion success, occlusion, phlebitis | 2022 | RCT5 | Journal of Hospital Medicine | Rickard CM, Larsen E, Walker RM, et al. |
| Nexiva™ | Smiths Jelco ProtectIV® Plus | Clinician satisfaction, dwell time, infection, infiltration, pain, phlebitis | 2019 | Prospective, quasi-experimental6 | Journal of Infusion Nursing | Penoyer D, Fowler S, Bennett M |
| Nexiva™ | None | Blood exposure, dislodgement, kinking, leaking, phlebitis, restarts | 2017 | Observational7 | Journal of Vascular Access | deRosenroll A |
| Nexiva™ | Medikit | Bending and kinking, blood exposure, cost savings/utilization reduction, dislodgement, dwell time, extravasation, insertion success, obstruction, phlebitis | 2014 | Quasi-randomized study8 | Journal of Vascular Access | Tamura N, Abe S, Hagimoto K, et al. |
| Nexiva™ | B. Braun Vasocan® Safety | Catheter-related infection, cost savings/utilization reduction, dwell time, infiltration/extravasation, insertion success, occlusion, pain, painful hematoma, phlebitis | 2014 | RCT9 | Journal of Vascular Access | Tamura N, Abe S, Hagimoto K, et al. |
| Nexiva™ | B. Braun Introcan Safety® (non-winged) with StatLock™ | Clinician satisfaction, cost savings/utilization reduction, dislodgement, dwell time, infiltration, insertion success, leaking, phlebitis | 2010 | RCT10 | Journal of Infusion Nursing | Bausone-Gazda D, Lefaiver CA, Walters SA, et al. |
| Nexiva™ | None | Clinician satisfaction, clotting, dislodgement, infiltration, leaking, restarts, securement | 2009 | Pre-and post-use survey1 | Journal of the Association of Vascular Access | McNeil E, Hines N, Phariss R |
| Nexiva Diffusics™ | Smiths Jelco ProtectIV® Plus | Aortic enhancement levels, catheter placement success, complications, contrast volume, extravasation, image quality, infusion rate, maximum pressure | 2014 | RCT with super script11 | American Journal of Roentgenology | Johnson PT, Christensen GM, Fishman EK. |
- McNeil E, Hines N, Phariss R. A clinical trial of a new all-in-one peripheral-short catheter. JAVA. 2009;14(1):46-51.
- Gidaro A, Quici M, Giustivi D, et al. Integrated short peripheral intravenous cannulas and risk of catheter failure: a systematic meta-analysis. J Vasc Access. 2024.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2024.
- Gerceker GO, Yildirim BG, Onal A, et al. The effect of the closed intravenous catheter system on first insertion success, indwelling time, and complications in pediatric hematology and oncology patients: a randomized controlled study. Eur J Oncol Nurs. 2023 Dec:67:102430. doi: 10.1016/j.ejon.2023.102430. Epub 2023 Oct 7.
- Rickard CM, Larsen E, Walker Rachel M, et al. Integrated versus nonintegrated peripheral intravenous catheter in hospitalized adults (OPTIMUM): A randomized controlled trial. J Hosp Med. 2022;1-12.
- Penoyer D, Fowler S, Bennett M. Evaluation of the Use of Open Versus Closed Short Peripheral Catheters on Catheter Dwell Time. J Infus Nurs. 2019;42(6):276-282.
- deRosenroll A. Peripheral intravenous catheters: Improving outcomes through change in products, clinical practice and education. Vasc Access. 2017;11(1):7-12.
- Tamura N, Abe S, Hagimoto K, et al. Unfavorable peripheral intravenous catheter replacements can be reduced using an integrated closed intravenous catheter system. J Vasc Access. 2014;15(4):257-263.
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- Johnson PT, Christensen GM, Fishman EK. IV contrast administration with dual source 128-MDCT: a randomized controlled study comparing 18-gauge nonfenestrated and 20-gauge fenestrated catheters for catheter placement success, infusion rate, image quality and complications. Am J Roentgenol. 2014;202(6):1166-70.
BD-117237 5/24