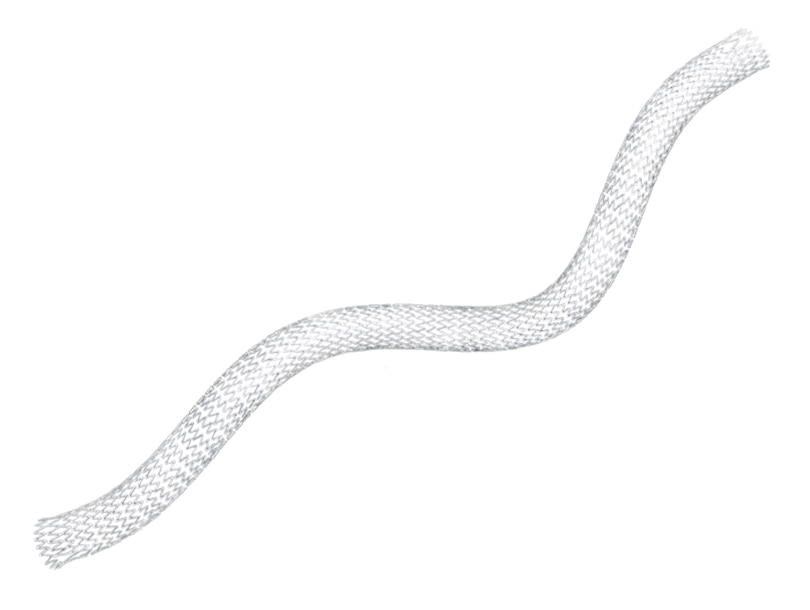
Proven performance. The LifeStent™ Vascular Stent is FDA-approved stent for the SFA and full popliteal artery.
Long term sustained effectiveness up to three years, and treatment superiority over balloon angioplasty.
Engineered for bending, compression, and torsion with dynamic vessel conformability.
Available in 6 mm and 7 mm diameters; and 150 mm to 170 mm in length.
Additionally, the LifeStent™ Solo™ Vascular Stent is available in 6 mm and 7 mm diameters and 200 mm in length.