*Compared to an open system.
**Results and savings may vary for other institutions.
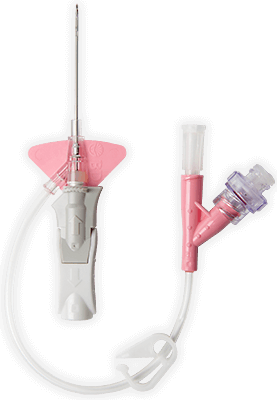
†Compared with an FEP catheter.
‡When used with a BD Nexiva™ specially designed 3M™ Tegaderm™ IV site securement dressing.
§Compared with B. Braun Introcan Safety® catheter with Bard Statlock® IV Ultra stabilization device.
||Compared with 96 hours in an open system.
¶Compared with a non-blood control catheter.
References
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Ann Intern Med. 1991;114(10):845-854.
- Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. 2021, Infusion Therapy Standards of Practice, 8th Edition. Journal of Infusion Nursing. Section 6 (S108). https://doi.org/10.1097/nan.0000000000000396
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16.
BD-37378 (09/2021)