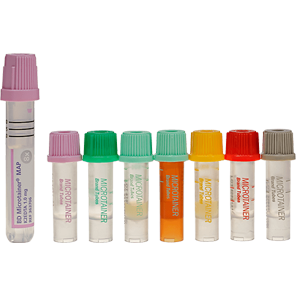
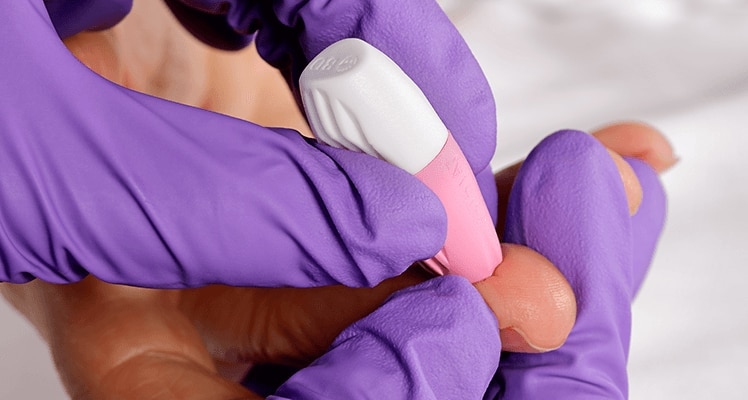
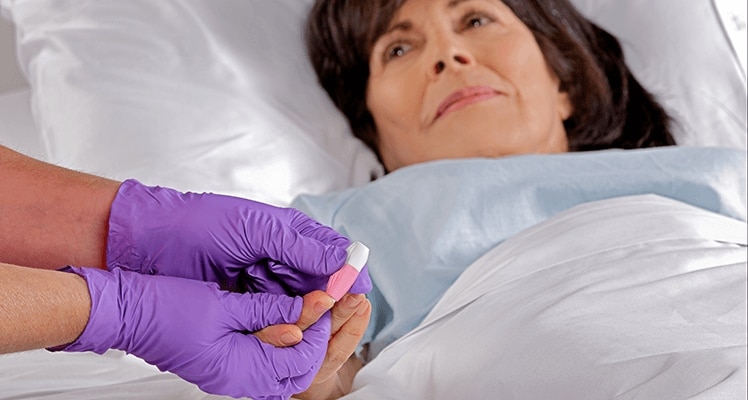
The BD Microtainer® Contact-Activated Lancet is a safety-engineered device used for the fingerstick collection of blood. Fingersticks should not be performed on children less than 1 year of age (according to CLSI guidelines*).


- Overview
- EIFU & Resources


Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
This is intended for Health Care Professionals only.
Notes
* Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS): Procedures and Devices for the Collection of Diagnostic Blood Specimens by Skin Puncture; Approved Standard (Sixth Edition). Wayne, PA, USA, 2008: Document H4-A6.
This medical device is CE marked in compliance with European Medical Device Directive 93/42/EEC and is CE certified by NSAI - National Standards Authority of Ireland (Notify Body Number = 0050 for BD Microtainer® Contact-Activated Lancet (CAL) and BD Microtainer® Quikheel™ Lancet)
BD-18634