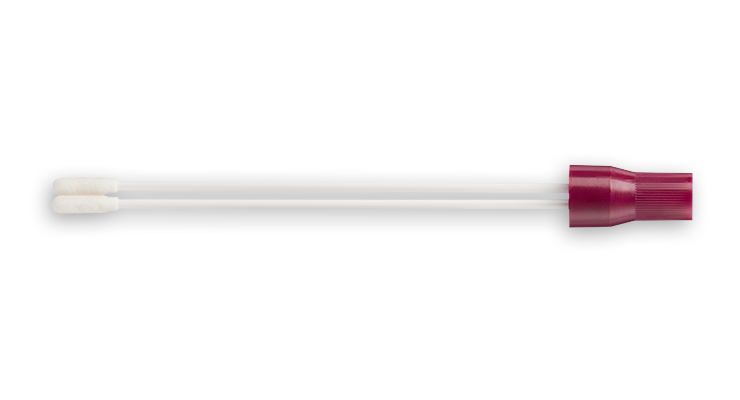
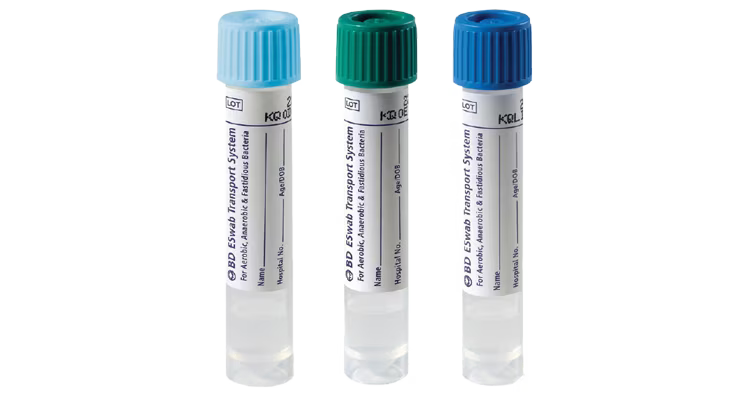
The BD ESwab® collection and transport system helps collect clinical specimens containing aerobic, anaerobic and fastidious bacteria from the collection site, and transport them to the testing laboratory. In the lab, BD ESwab® specimens are processed using standard clinical lab operating procedures for bacterial culture. The unique design of the flocked swab ensures optimal elution of the specimen into the transport medium.

- Overview
- EIFU & Resources
The BD ESwab® system is 1 mL of modified liquid Amies medium packaged with a nylon flocked swab.
The BD ESwab® system provides 1 mL of sample suspension for Gram stain and multiple culture analyses.

BD CultureSwab™ MaxV collection and transport systems
ESwab is a registered trademark of COPAN ITALIA S.P.A.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017