Therapeutic and Clinical Areas
Content Type
Product Brand
Product Family
New Survey Reveals Nearly 3 in 4 Canadian Women Are Delaying Their OB/GYN Exams, Increasing Risks for Cervical Cancer
BD announced the results of a new survey revealing that 74% of women in Canada have delayed a gynecology visit, with 83% wanting more accessible and less invasive cervical cancer testing options, including at-home self-collection for human papillomavirus (HPV) tests.
Women In Canada Can Now Collect Their Own Sample At Home For Cervical Cancer Screening
BD announced today Health Canada approval of the BD Onclarity™ HPV Assay for human papillomavirus (HPV) testing for the use of self-collected vaginal specimens at home.
Mackenzie Health launches first-of-its-kind technology in Canada designed to help reduce IV pump programming medication errors
Mackenzie Health recently launched BD Alaris™ EMR Bidirectional Interoperability, which allows for two-way information flow between an IV pump and a patient’s electronic medical record.
Humber and BD seek to address health human resource challenges
Humber and BD are working together to reshape the landscape of health care workforce development and address the evolving needs of the industry along with health human resources.
Statement on US FDA Safety Communication
BD-Canada is aware of the U.S. FDA issuing Safety Communication stating concern that certain plastic syringes may not provide consistent and adequate quality or performance.
Health Inequity: Tackling the Profound Impact of Chronic Kidney Disease on Indigenous Peoples
The kidney care community advocates for more cost-effective treatment options, which offer a better quality of life and improved outcomes, although several obstacles hinder significant progress in improving kidney failure treatment.
Health Inequity: Bold Action and Collaboration Needed to Eradicate Cervical Cancer in Canada
Cervical cancer is one of the few cancers that is almost completely preventable through systematic screening programs, early detection with HPV testing and vaccination. Our generation holds the incredible potential to seize the opportunity to eradicate cervical cancer within our lifetime.
London’s hospitals are leading the way in clinical microbiology with a world-first installation of state-of-the-art automated laboratory technology
London Health Sciences Centre (LHSC) and St. Joseph’s Heath Care London (St. Joseph’s) are celebrating the installation of a Total Laboratory Automation (TLA) system within the clinical microbiology laboratory.
BD Awards Educational Grant for Point of Care Testing Pilot in Alberta
The program led by 19 to Zero aims to support point-of-care testing needs beyond COVID-19 for urban and rural communities in Alberta through education and diagnosis at clinics.
BD Receives Health Canada Authorization for COVID-19, Influenza A/B, RSV Combination Test
BD announced that it had received Authorization under the Interim Order from Health Canada for a new molecular diagnostic combination test for SARS-CoV-2, Influenza A and B and Respiratory Syncytial Virus (RSV) to help combat a potential “tripledemic” in the upcoming respiratory virus season.
Therapeutic and Clinical Areas
Product Brand
Product Family
Product Category
Product Line
Product Segment
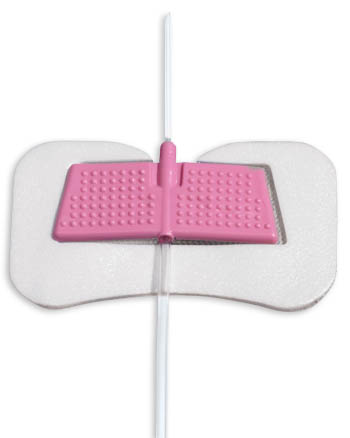


BD offers stabilization devices for latex and silicone catheters. They are available in adult and pediatric sizes.
SKU: vfdssp
-

The PureWick™ Female External Catheter allows for simple, non-invasive urine output management in female patients.
-

Provides a modular platform you can build on to customise infusion delivery based on patient needs.
-

BD ChloraPrep™ Sterile Solution has a full portfolio of products to meet the coverage area needs of each patient: 3mL, 10.5 mL, and 26 mL sizes.
-

BD Microtainer® Blood Collection Tubes are non-sterile, single use in vitro diagnostic medical devices.
-

WavelinQ™ EndoAVF System for endovascular AV fistula creation.
-

The Gel Mark UltraCor™ Breast Tissue Marker features a 14G rigid needle with a bevelled tip.
-
Experience the efficiency of power injection for CECT scans and PICC therapy with PowerPICC™ catheters.
- BD Biosciences Inquiries
- Distributor or Wholesale Inquiries
Distributor or Wholesale Inquiries
bdcscanada@bd.com
BD - Canada 2100 Derry Road West Suite 100 Mississauga, ON L5N 0B3 Support
Content Type
Product Brand
Product Family
- LAST UPDATE: Dec. 23, 2024Instructions for UsePrescriptive information
- LAST UPDATE: Mar. 06, 2024Instructions for UseLubricious Double Pigtail Ureteral Stent with Suture and Multi-Length Lubricious Ureteral Stent with Suture
- LAST UPDATE: Mar. 06, 2024Instructions for UseThe ureteral catheter should be inserted into the ureter using standard endourologic technique, under direct, fluoroscopic or radiographic visualization.
- LAST UPDATE: Mar 11, 2024BrochureBD’s extensive line of sterile patient preoperative skin preparation products raises the standard.
- LAST UPDATE: Mar 08, 2024BrochurePhasix™ Mesh family highlights
- LAST UPDATE: Mar 11, 2024Quick GuideDiscover More About Deep Venous Disease & Optimize Your Treatment Options in this Patient Guide
- LAST UPDATE: Mar 12, 2024IllustrationFront and back view of venous anatomy in the leg
- LAST UPDATE: Mar 12, 2024Patient EducationCould your leg pain be related to chronic venous disease?
- LAST UPDATE: Jun 19, 2024License AgreementMaster Purchase, License And System Support Terms & Conditions Synergy Medical BRG Inc.