Uncoated medium weight monofilament polypropylene mesh on the anterior side with an absorbable hydrogel barrier based on Sepra™ Technology on the posterior side for laparoscopic ventral hernia repair.
Ventralight™ ST Mesh
Uncoated medium weight monofilament polypropylene mesh on the anterior side with an absorbable hydrogel barrier based on Sepra® Technology on the posterior side for laparoscopic ventral hernia repair.


- Overview
- Products & Accessories
- EIFU & Resources
Efficient
- Low profile design facilitates trocar deployment and mechanical fixation.
- Multiple shapes (circle, oval, ellipse, rectangle) and sizes ranging from a 4.5" (11.4 cm) circle to 12" x 14" (30.5 cm x 35.6 cm) rectangle.
- Easily cut to customise shape and size. The unique hydrogel barrier covers the edge of the mesh even after trimming.
Effective
Minimal contraction shown in preclinical testing compared to a leading microporous absorbable barrier mesh
At 4 weeks, Ventralight™ ST Mesh demonstrated 42% less area meshcontracture than a competitive macroporous absorbable barrier mesh.
Results were statistically significant.*
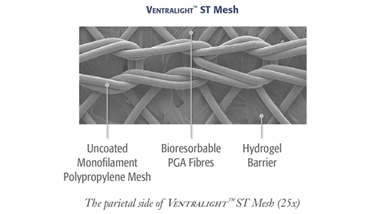
Fibres
Ventralight™ Absorbable Barrier based on Sepra® Technology
- Unique hydrogel barrier swells to minimise tissue attachment to the visceral side of the mesh*
- The hydrogel barrier resorbs within 30 days providing visceral protection during the critical healing period*
Strong tissue Proven incorporation
The open-pore design of the uncoated monofilament polypropylene in Ventralight™ ST Mesh allows for:
- Fast tissue ingrowth
- Strong tissue incorporation into the abdominal wall
- A strong, long-term repair uncoated polypropylene allows for the majority of tissue ingrowth and strength to occur in the first two weeks after the placement of a composite hernia prosthesis.**
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies in the repair of ventral, incisional and umbilical hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach. This mesh is not for the use of treatment of stress urinary incontinence.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
- This mesh is not for the use of treatment of stress urinary incontinence.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies in the repair of ventral, incisional and umbilical hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach. This mesh is not for the use of treatment of stress urinary incontinence.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
- This mesh is not for the use of treatment of stress urinary incontinence.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies in the repair of ventral, incisional and umbilical hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach. This mesh is not for the use of treatment of stress urinary incontinence.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
- This mesh is not for the use of treatment of stress urinary incontinence.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
