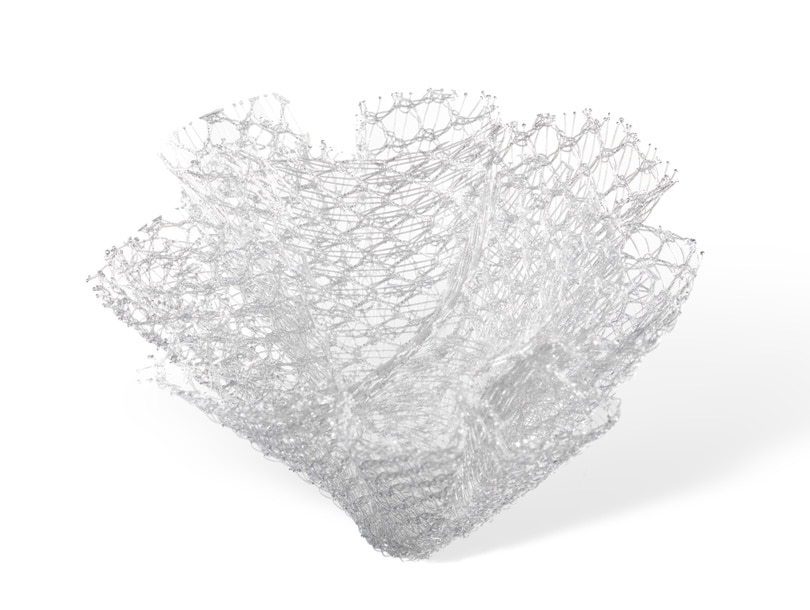
PerFix™ Plug
Plug and patch designed for use in a tension-free open inguinal hernia repair technique.


- Overview
- EIFU & Resources
A quick and simple preperitoneal underlay Modified Technique for the repair of groin hernias
The PerFix™ Plug is ideal for use in a tension-free preperitoneal repair technique. With over five million implants worldwide, the Bard™ PerFix™ Plug is designed with pleated edges that conform readily to defects of various sizes and shapes. The monofilament polypropylene design ensures healthy tissue ingrowth.

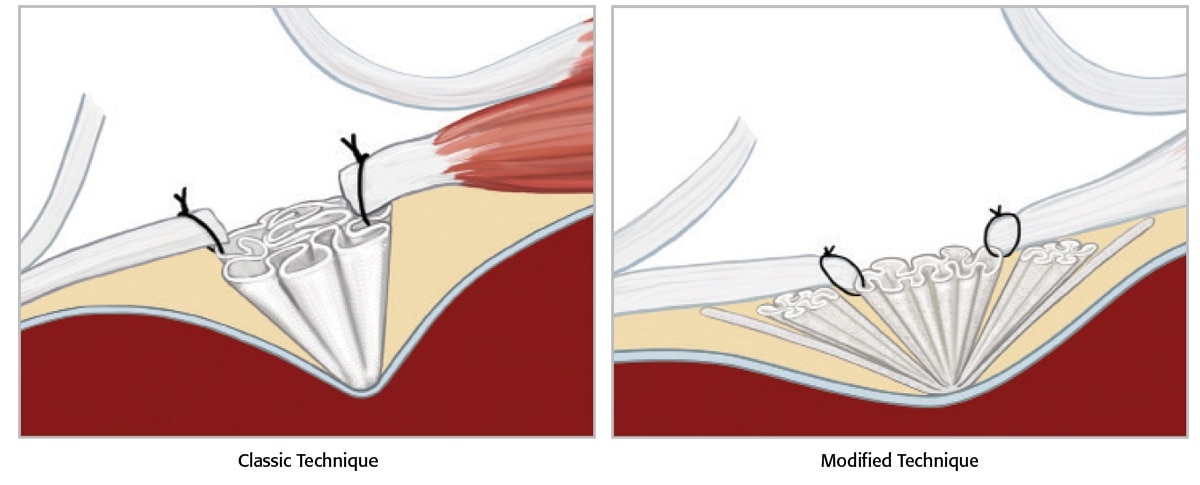
Entire operation can take 25 minutes or less2 Minimal dissection is required 4-5cm incision1 Local or epidural anaesthesia can be used Accommodates all types of groin hernias
Utilised in a tension-free repair technique Recurrence rates reported at 0.15%1 Less than 0.5% chronic pain rate1
1. Millikan KW, Doolas A. A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients. Hernia. 2008 June; 12(3): 257-260.
2. Rutkow, I.M. : The Perfix plug repair for groin hernias. Surg Clinics of N. America, 2003; 83; 5: 1079-1098; 78; 6: 1007-1023
INDICATIONS
This product is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal and femoral hernias.
CONTRAINDICATIONS
Literature reports that there may be a possibility for adhesion formation when Bard® mesh is placed in direct contact with the bowel or viscera.
Do not use the PerFix™ Plug in infants or children, whereby future growth will be compromised by use of such mesh material.
WARNINGS
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
This device is not for the use of repair of pelvic organ prolapse. This device is not for the use of treatment of stress urinary incontinence
PRECAUTIONS
Monofilament sutures are recommended to properly secure the PerFix™ Plug
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
1. Millikan KW, Doolas A. A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients. Hernia. 2008 June; 12(3): 257-260.
2. Rutkow, I.M. : The Perfix plug repair for groin hernias. Surg Clinics of N. America, 2003; 83; 5: 1079-1098; 78; 6: 1007-1023
INDICATIONS
This product is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal and femoral hernias.
CONTRAINDICATIONS
Literature reports that there may be a possibility for adhesion formation when Bard® mesh is placed in direct contact with the bowel or viscera.
Do not use the PerFix™ Plug in infants or children, whereby future growth will be compromised by use of such mesh material.
WARNINGS
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
This device is not for the use of repair of pelvic organ prolapse. This device is not for the use of treatment of stress urinary incontinence
PRECAUTIONS
Monofilament sutures are recommended to properly secure the PerFix™ Plug
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.