1 LeBlanc K. “Proper mesh overlap is a key determinant in hernia recurrence following laparoscopic ventral and incisional hernia repair“ Hernia 2016 Feb;20(1):85-99.
2 Liang MK, Clapp ML, Garcia A, Subramanian A, Awad SS. “Mesh shift following laparoscopic ventral hernia repair.” J Surg Res. 2012 Sep;177(1):e7-13.
3 Tollens T, Topal H, Ovaere S, Beunis A, Vermeiren K, Aelvoet C. “Prospective analysis of ventral hernia repair using the Ventralight™ ST hernia patch.” Surg Technol Int. 2013 Sep;23:113-6.
4 References available upon request.
Indications
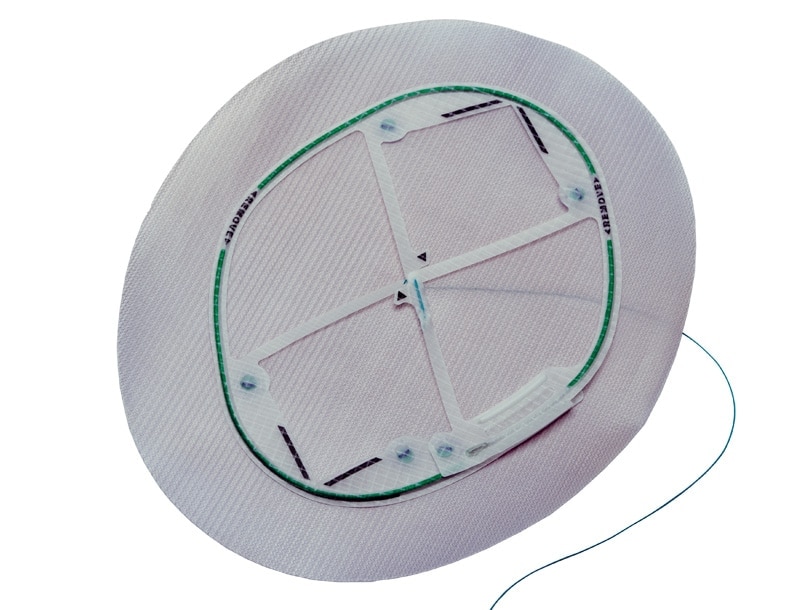
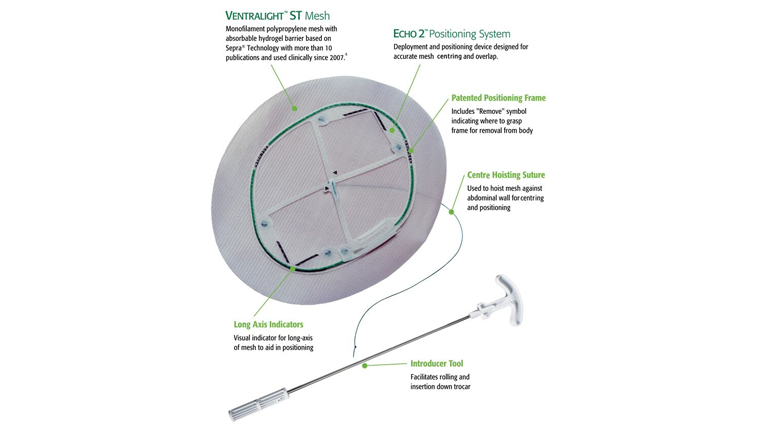
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies in the repair of ventral, incisional, and umbilical hernias. The Echo 2™ Positioning System is intended to facilitate the delivery and positioning of the Ventralight™ ST Mesh during laparoscopic hernia repair.
Contraindications
- Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by the use of such mesh materials.
- The use of this mesh has not been studied in breastfeeding or pregnant women.
- Do not use this mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
Warnings
- The use of any permanent mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
- If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections.
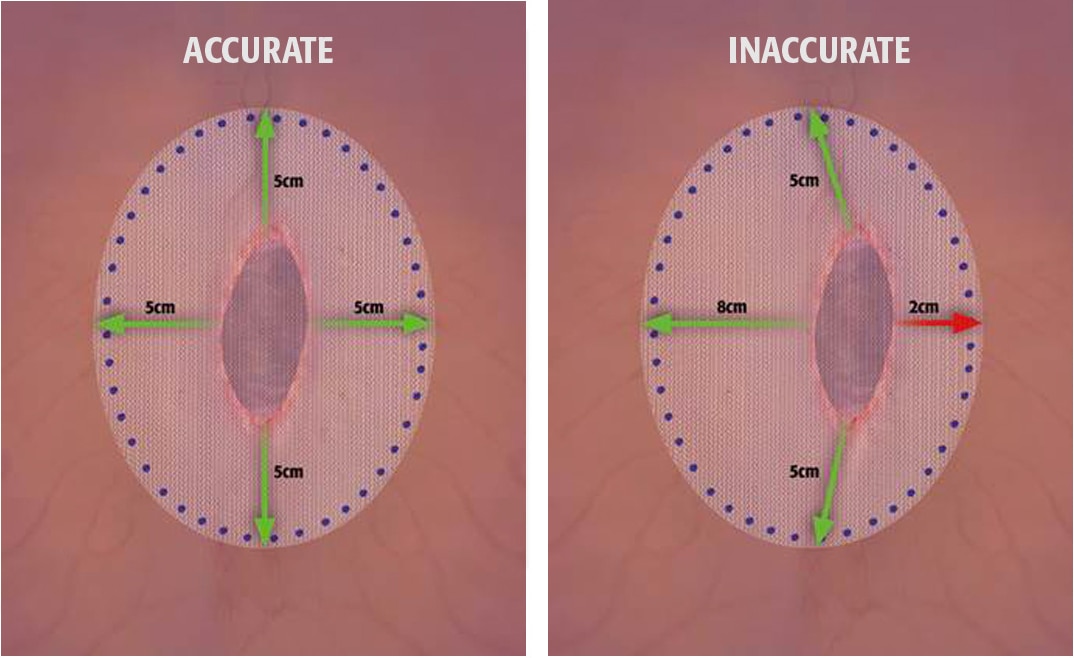
- To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation, placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
- This device is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
- This device is designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user.
- This mesh should be used once the exterior foil pouch has been opened. Do not store for later use. Unused portions of the mesh should be discarded.
- Ensure proper orientation; the coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera (see “Surface Orientation”).
- Do not apply sharp, pointed, cautery devices, or ultrasonic tools (such as scissors, needles, tackers, diathermic tools, etc.) to the Echo 2™ Positioning System frame.
- This device contains superelastic nitinol wire; do not cut and avoid direct contact/coupling with active surgical electrodes.
- Ventralight™ ST Mesh is the only permanent implant component of the device. The Echo 2™ Positioning System (which includes deployment frame, center hoisting suture and all connectors) must be removed from the patient and appropriately discarded. It is not part of the permanent implant.
- The Echo 2™ Positioning System should not be used with any other hernia mesh aside from those with which it comes pre-attached/packaged.
- Discard the Echo 2™ Positioning System (including the frame, center hoisting suture, all connectors and Mesh Introducer) after use. These may be a potential biohazard. Handle and dispose in accordance with accepted medical practice and applicable local, state and federal laws and regulations.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
- This mesh is not for the use of treatment of stress urinary incontinence.
Precautions
- Please read all instructions prior to use.
- Only physicians qualified and trained in the appropriate surgical techniques should use this device.
- The safety and effectiveness of the device has not been evaluated in clinical studies for the presence of malignancies in the abdominopelvic cavity.
- Visualization must be maintained throughout the course of the entire procedure. Additionally, laparoscopic removal of the Echo 2™ Positioning System must be performed under sufficient visualization of the entire device and surrounding anatomy to ensure proper removal.
- Do not trim the mesh. This will affect the interface between the mesh and the positioning system.
Adverse Reactions
Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction, and recurrence of the hernia or soft tissue defect.