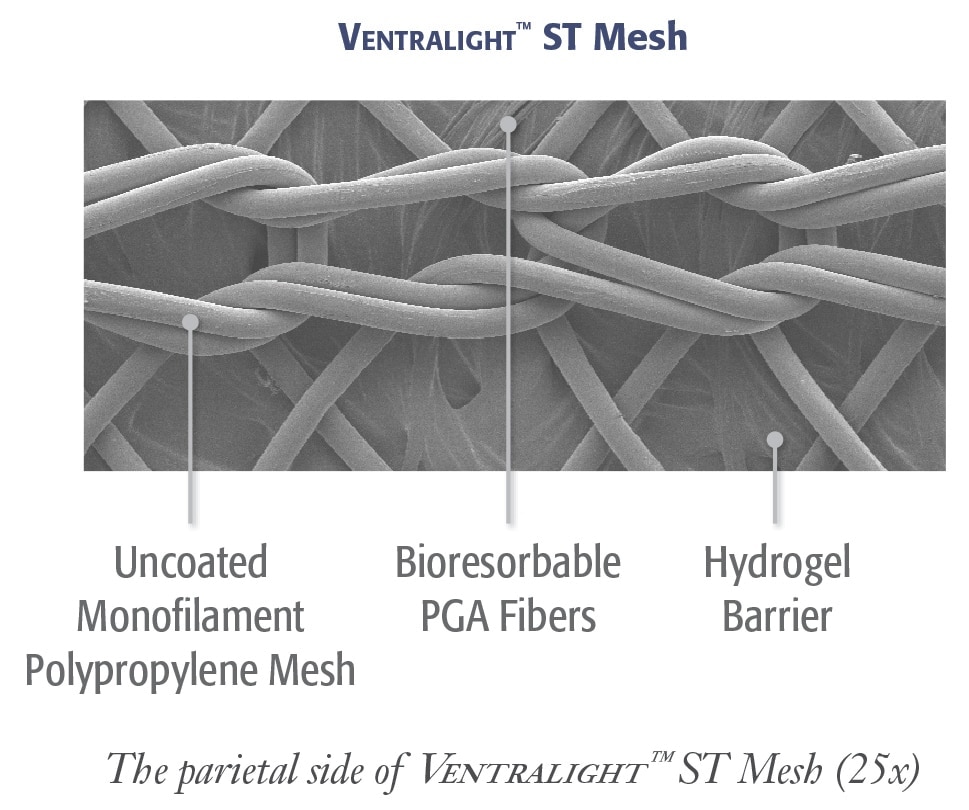
복강경 복부 탈장 수복을 위하여 코팅되지 않은 중량 모노필라멘트 폴리프로필렌 메쉬는 전방 면에, Sepra® 기술에 기반한 흡수성 하이드로젤 ® 장벽은 후방 면에 있습니다.


- 개요
- 제품 및 부속품
- EIFU 및 리소스
효율적입니다
- 로우 프로파일 설계로 투관침 배치 및 기계적 고정이 용이합니다.
- 여러 모양(원형, 계란형, 타원형, 직사각형)과 4.5인치(11.4cm) 원부터 12인치x14인치(30.5cmx35.6cm) 직사각형까지 다양한 크기를 제공합니다.
- 모양과 크기를 원하는 대로 쉽게 자를 수 있습니다. 트리밍 후에도 고유한 하이드로젤 장벽이 메쉬의 가장자리를 덮어줍니다.
효과적입니다
앞선 미소공성 흡수성 장벽 메쉬와 비교한 전임상 시험에서 최소 수축 입증
4주 후 Ventralight™ ST 메쉬는 경쟁사의 다공성 흡수성 장벽 메쉬보다 면적이 42% 적은 메쉬 수축을 보여줬습니다.
결과는 통계적으로 유의미했습니다.*
입증됐습니다
Sepra® 기술에 기반한 Ventralight™ 흡수성 장벽
- 메쉬의 내장면에 조직이 유착되는 것을 최소화하기 위한 고유한 하이드로젤 장벽 팽창 기능*
- 30일 이내 하이드로젤 장벽 흡수로 중요한 치유 기간 동안 내장 보호 제공*
입증된 강력한 조직 결합
Ventralight™ ST 메쉬의 비코팅 모노필라멘트 폴리프로필렌의 개방형 기공 설계는 다음을 가능하게 합니다.
- 빠른 조직 내성장
- 복벽에 강력한 조직 결합
- 강력하고 장기적인 수복
- 비코팅 폴리프로필렌은 복합 탈장 보철물 삽입 후 첫 2주 동안 대부분의 조직이 성장하고 강도가 높아지게 합니다.**
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-126372
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
