쉽습니다. 효율적입니다. 탈장 수복에 대해 입증됐습니다.
SorbaFlex™ 메모리 기술과 Sepra® 기술을 적용한 흡수성 장벽을 갖춘 임상적으로 입증된 배꼽 탈장 수복 솔루션.
리히터 탈장과 투관침 부위 탈장에 적합합니다. 가장 작은 Ventralex™ ST 탈장 패치를 사용하면 경근막 봉합 없이 복강내 무장력 수복을 할 수 있습니다.
4.3cm, 6.4cm, 8cm를 제공합니다.


쉽습니다. 효율적입니다. 탈장 수복에 대해 입증됐습니다.
SorbaFlex™ 메모리 기술과 Sepra® 기술을 적용한 흡수성 장벽을 갖춘 임상적으로 입증된 배꼽 탈장 수복 솔루션.
리히터 탈장과 투관침 부위 탈장에 적합합니다. 가장 작은 Ventralex™ ST 탈장 패치를 사용하면 경근막 봉합 없이 복강내 무장력 수복을 할 수 있습니다.
4.3cm, 6.4cm, 8cm를 제공합니다.

Ventralex™ ST 탈장 패치의 전방층
메쉬의 틈을 통해 섬유아세포가 신속하게 반응할 수 있도록 설계된 모노필라멘트 폴리프로필렌 메쉬.
강력한 탈장 수복을 위한 설계
폴리디옥사논(PDO) 모노필라멘트는 고유한 유연성과 장력 강도로 패치 삽입 및 배치를 용이하게 합니다. 전임상 시험 결과 가수분해를 통한 흡수는 기본적으로 6~8개월 안에 완전히 흡수되는 것으로 드러났습니다.4
포켓과 스트랩은 배치, 위치 조정 및 측면 고정을 용이하게 합니다. SorbaFlex™ 메모리 기술은 니트 폴리프로필렌 메쉬 튜브에 포함되어 있습니다.
탈장 패치의 후방층
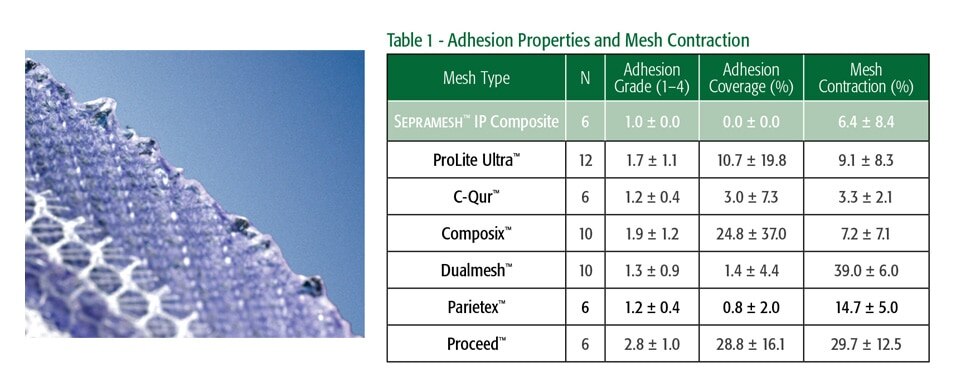
Sepramesh™ IP 컴포지트는 Sepra® 기술에 기반을 둔 고유한 하이드로젤 장벽으로, 팽창하면서 내장 쪽 메쉬에 조직이 부착되는 것을 최소화하고 30일 이내에 재흡수되어 중요한 치유 과정 동안 내장을 보호합니다.3
생체 재흡수성 PGA 섬유는 폴리프로필렌 메쉬와 결합하여 하이드로젤 장벽의 무결성을 강화합니다.
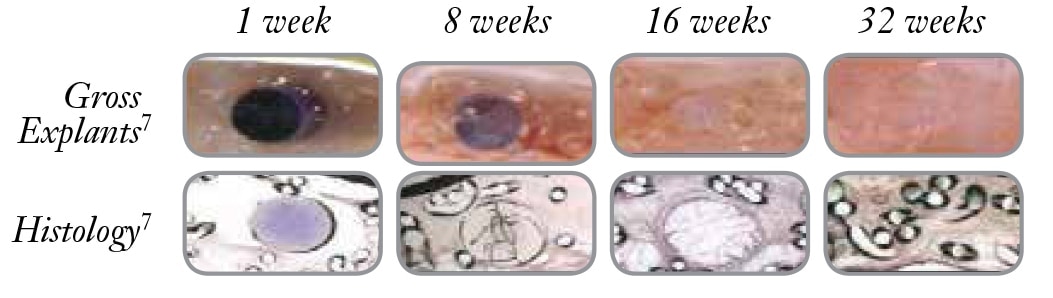
복강 내 배치 후 새로운 오메가-3 지방산 생체 흡수성 장벽 다공성 메쉬에 대한 접착 등급 및 양, 메쉬 수축 및 조직 반응 120일 비교 분석.
Ventralex™ ST 탈장 패치 장점
1 Majercik, S. et al. “Strength of tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endos. (2006) 20: 1671-1674.
2 Results may not correlate to performance in humans.
3 Pierce, Richard A. MD, PhD, et al. Surgical Innovation. March 2009; 16, 1:45-54.
4 Preclinical data on file at C. R. Bard. Results may not correlate to performance in humans.
5 These images are from a porcine study using the Ventrio™ Hernia Patch which contains the same SorbaFlex™ Memory Technology
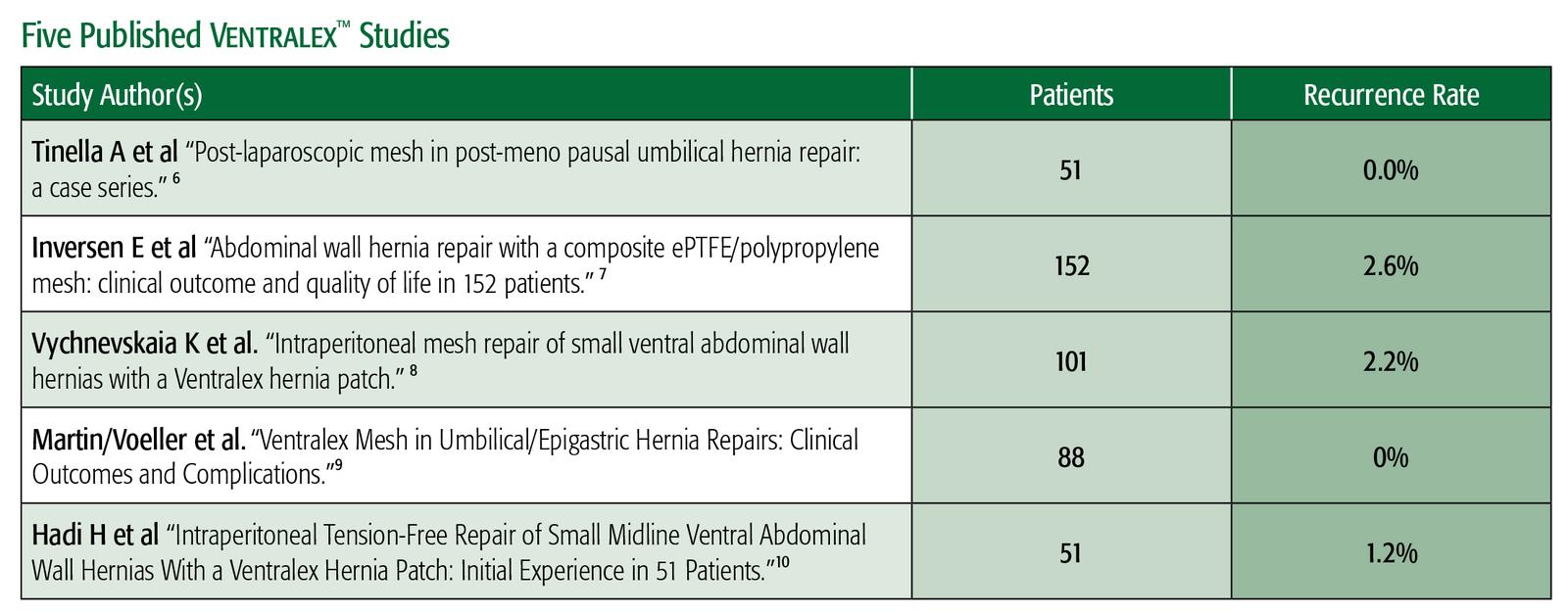
6 Tinella A, Malvasi A, Manca C, Alemanno G, Bettocchi S, Benhidjeb T. “Post-laparoscopic mesh in post-menopausal umbilical hernia repair: a case series.” Minim Invasive Ther Allied Technol. 2011 Sep; 20(5):290-5.
7 Iversen E, Lykke A, Hensler M, Jorgensen LN. “Abdominal wall hernia repair with a composite ePTFE/polypropylene mesh: clinical outcome and quality of life in 152 patients.” Hernia. 2010 Dec;14(6): 555-60.
8 Vychnevskaia K, Mucci-Hennekinne S, Casa C, et al. “Intraperitoneal mesh repair of small ventral abdominal wall hernias with a Ventralex™ Hernia Patch.” Dig Surg. 2010; 27(5): 433-5.
9 D.F. Martin, R.F. Williams, T. Mulrooney, and G.R. Voeller. “Ventralex™ Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications.” Hernia. 2008 Aug 12(4) 379-83.
10 H.I.A. Hadi, A. Maw, S. Sarmah, P. Kumar. “Intraperitoneal Tension-Free Repair of Small Midline Ventral Abdominal Wall Hernias With a Ventralex™ Hernia Patch: Initial Experience in 51 Patients.” Hernia. 2006: 10:409-413.
INDICATIONS
The Ventralex™ ST Hernia Patch is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, including repair of hernias and deficiencies caused by trocars.
CONTRAINDICATIONS
Do not use the Ventralex™ ST Hernia Patchin infants or children, whereby future growth will be compromised by the use of such mesh material. Do not use the Ventralex™ ST Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape the Ventralex™ ST Hernia Patch, as this could impact its effectiveness, except for the polypropylene positioning strap. Care should be taken not to cut or nick the SorbaFlex™PDO monofilament during insertion or fixation. If the SorbaFlex™PDO monofilament is cut or damaged, additional complications may include bowel or skin perforation and infection. Follow proper folding techniques for all patches as described in these Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament. Ensure proper orientation; the bioresorbable coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh side against the bowel. There may be a possibility for adhesion formation when the mesh is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
BD-126368
1 Majercik, S. et al. “Strength of tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endos. (2006) 20: 1671-1674.
2 Results may not correlate to performance in humans.
3 Pierce, Richard A. MD, PhD, et al. Surgical Innovation. March 2009; 16, 1:45-54.
4 Preclinical data on file at C. R. Bard. Results may not correlate to performance in humans.
5 These images are from a porcine study using the Ventrio™ Hernia Patch which contains the same SorbaFlex™ Memory Technology
6 Tinella A, Malvasi A, Manca C, Alemanno G, Bettocchi S, Benhidjeb T. “Post-laparoscopic mesh in post-menopausal umbilical hernia repair: a case series.” Minim Invasive Ther Allied Technol. 2011 Sep; 20(5):290-5.
7 Iversen E, Lykke A, Hensler M, Jorgensen LN. “Abdominal wall hernia repair with a composite ePTFE/polypropylene mesh: clinical outcome and quality of life in 152 patients.” Hernia. 2010 Dec;14(6): 555-60.
8 Vychnevskaia K, Mucci-Hennekinne S, Casa C, et al. “Intraperitoneal mesh repair of small ventral abdominal wall hernias with a Ventralex™ Hernia Patch.” Dig Surg. 2010; 27(5): 433-5.
9 D.F. Martin, R.F. Williams, T. Mulrooney, and G.R. Voeller. “Ventralex™ Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications.” Hernia. 2008 Aug 12(4) 379-83.
10 H.I.A. Hadi, A. Maw, S. Sarmah, P. Kumar. “Intraperitoneal Tension-Free Repair of Small Midline Ventral Abdominal Wall Hernias With a Ventralex™ Hernia Patch: Initial Experience in 51 Patients.” Hernia. 2006: 10:409-413.
INDICATIONS
The Ventralex™ ST Hernia Patch is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, including repair of hernias and deficiencies caused by trocars.
CONTRAINDICATIONS
Do not use the Ventralex™ ST Hernia Patchin infants or children, whereby future growth will be compromised by the use of such mesh material. Do not use the Ventralex™ ST Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape the Ventralex™ ST Hernia Patch, as this could impact its effectiveness, except for the polypropylene positioning strap. Care should be taken not to cut or nick the SorbaFlex™PDO monofilament during insertion or fixation. If the SorbaFlex™PDO monofilament is cut or damaged, additional complications may include bowel or skin perforation and infection. Follow proper folding techniques for all patches as described in these Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament. Ensure proper orientation; the bioresorbable coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh side against the bowel. There may be a possibility for adhesion formation when the mesh is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
BD-126368
1 Majercik, S. et al. “Strength of tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endos. (2006) 20: 1671-1674.
2 Results may not correlate to performance in humans.
3 Pierce, Richard A. MD, PhD, et al. Surgical Innovation. March 2009; 16, 1:45-54.
4 Preclinical data on file at C. R. Bard. Results may not correlate to performance in humans.
5 These images are from a porcine study using the Ventrio™ Hernia Patch which contains the same SorbaFlex™ Memory Technology
6 Tinella A, Malvasi A, Manca C, Alemanno G, Bettocchi S, Benhidjeb T. “Post-laparoscopic mesh in post-menopausal umbilical hernia repair: a case series.” Minim Invasive Ther Allied Technol. 2011 Sep; 20(5):290-5.
7 Iversen E, Lykke A, Hensler M, Jorgensen LN. “Abdominal wall hernia repair with a composite ePTFE/polypropylene mesh: clinical outcome and quality of life in 152 patients.” Hernia. 2010 Dec;14(6): 555-60.
8 Vychnevskaia K, Mucci-Hennekinne S, Casa C, et al. “Intraperitoneal mesh repair of small ventral abdominal wall hernias with a Ventralex™ Hernia Patch.” Dig Surg. 2010; 27(5): 433-5.
9 D.F. Martin, R.F. Williams, T. Mulrooney, and G.R. Voeller. “Ventralex™ Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications.” Hernia. 2008 Aug 12(4) 379-83.
10 H.I.A. Hadi, A. Maw, S. Sarmah, P. Kumar. “Intraperitoneal Tension-Free Repair of Small Midline Ventral Abdominal Wall Hernias With a Ventralex™ Hernia Patch: Initial Experience in 51 Patients.” Hernia. 2006: 10:409-413.
INDICATIONS
The Ventralex™ ST Hernia Patch is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, including repair of hernias and deficiencies caused by trocars.
CONTRAINDICATIONS
Do not use the Ventralex™ ST Hernia Patchin infants or children, whereby future growth will be compromised by the use of such mesh material. Do not use the Ventralex™ ST Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape the Ventralex™ ST Hernia Patch, as this could impact its effectiveness, except for the polypropylene positioning strap. Care should be taken not to cut or nick the SorbaFlex™PDO monofilament during insertion or fixation. If the SorbaFlex™PDO monofilament is cut or damaged, additional complications may include bowel or skin perforation and infection. Follow proper folding techniques for all patches as described in these Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament. Ensure proper orientation; the bioresorbable coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh side against the bowel. There may be a possibility for adhesion formation when the mesh is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
BD-126368