-
PerFix™ Plug, Extra Large, 1.6" x 2.0" (4.1cm x 5.0cm), 2/case
SKU/REF 0112780
-
PerFix™ Plug, Large, 1.6" x 1.90" (4.1cm x 4.8cm), 2/case
SKU/REF 0112770
-
PerFix™ Plug, Medium, 1.3" x 1.55" (3.3cm x 3.9cm), 2/case
SKU/REF 0112760
-
PerFix™ Plug, Small, 1.0" x 1.35" (2.5cm x 3.4cm), 2/case
SKU/REF 0112750
true
지원
Becton, Dickinson and Company
(82.2) 080-340-3800
bd_korea@bd.com
영업팀에 연락해 주셔서 감사합니다!
영업 담당자가 곧 연락을 드립니다.
Address
Becton Dickinson Korea Co., Ltd. 한국 서울특별시 강남구 테헤란로 142 아크플레이스 16층 06236


- 개요
- 제품 및 부속품
- EIFU 및 리소스
서혜부 탈장 수복을 위한 빠르고 간단한 복막전 언더레이 수정 기술
PerFix™ Plug는 무장력 복막전 수복 기술에 사용하기에 적합합니다. Bard™ PerFix™ Plug 는 다양한 크기와 모양의 결손 부위에 쉽게 맞도록 주름진 가장자리로 설계되어 있습니다. 모노필라멘트 폴리프로필렌 설계는 건강한 조직 내성장을 보장합니다.

true
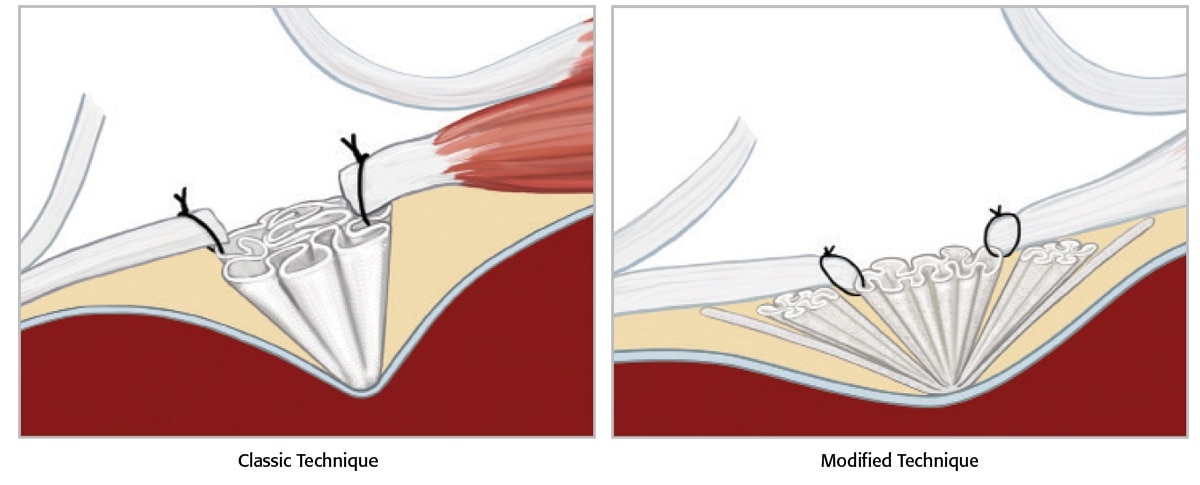
효율적입니다
25분 내로 전체 수술 가능2 최소 절개 필요 4~5cm 절개1 모든 국소 또는 경막외 마취 사용 가능 모든 유형의 서혜부 탈장 수용
효과적입니다
무장력 수복 기술 사용 보고된 재발률 0.15%1 만성 통증 발생률 0.5% 미만1
1. Millikan KW, Doolas A. A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients. Hernia. 2008 June; 12(3): 257-260.
2. Rutkow, I.M. : The Perfix plug repair for groin hernias. Surg Clinics of N. America, 2003; 83; 5: 1079-1098; 78; 6: 1007-1023
INDICATIONS
This product is indicated for the repair of groin hernia defects.
CONTRAINDICATIONS
Literature reports that there may be a possibility for adhesion formation when Perfix™ Plug or the Polypropylene mesh is placed in direct contact with the bowel or viscera.
Do not use the PerFix™ Plug in infants or children, whereby future growth will be compromised by use of such mesh material.
WARNINGS
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
PRECAUTIONS
Monofilament sutures are recommended to properly secure the PerFix™ Plug
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-126373
true
1. Millikan KW, Doolas A. A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients. Hernia. 2008 June; 12(3): 257-260.
2. Rutkow, I.M. : The Perfix plug repair for groin hernias. Surg Clinics of N. America, 2003; 83; 5: 1079-1098; 78; 6: 1007-1023
INDICATIONS
This product is indicated for the repair of groin hernia defects.
CONTRAINDICATIONS
Literature reports that there may be a possibility for adhesion formation when Perfix™ Plug or the Polypropylene mesh is placed in direct contact with the bowel or viscera.
Do not use the PerFix™ Plug in infants or children, whereby future growth will be compromised by use of such mesh material.
WARNINGS
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
PRECAUTIONS
Monofilament sutures are recommended to properly secure the PerFix™ Plug
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-126373
true
true
true
1. Millikan KW, Doolas A. A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients. Hernia. 2008 June; 12(3): 257-260.
2. Rutkow, I.M. : The Perfix plug repair for groin hernias. Surg Clinics of N. America, 2003; 83; 5: 1079-1098; 78; 6: 1007-1023
INDICATIONS
This product is indicated for the repair of groin hernia defects.
CONTRAINDICATIONS
Literature reports that there may be a possibility for adhesion formation when Perfix™ Plug or the Polypropylene mesh is placed in direct contact with the bowel or viscera.
Do not use the PerFix™ Plug in infants or children, whereby future growth will be compromised by use of such mesh material.
WARNINGS
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
PRECAUTIONS
Monofilament sutures are recommended to properly secure the PerFix™ Plug
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-126373
true
true
