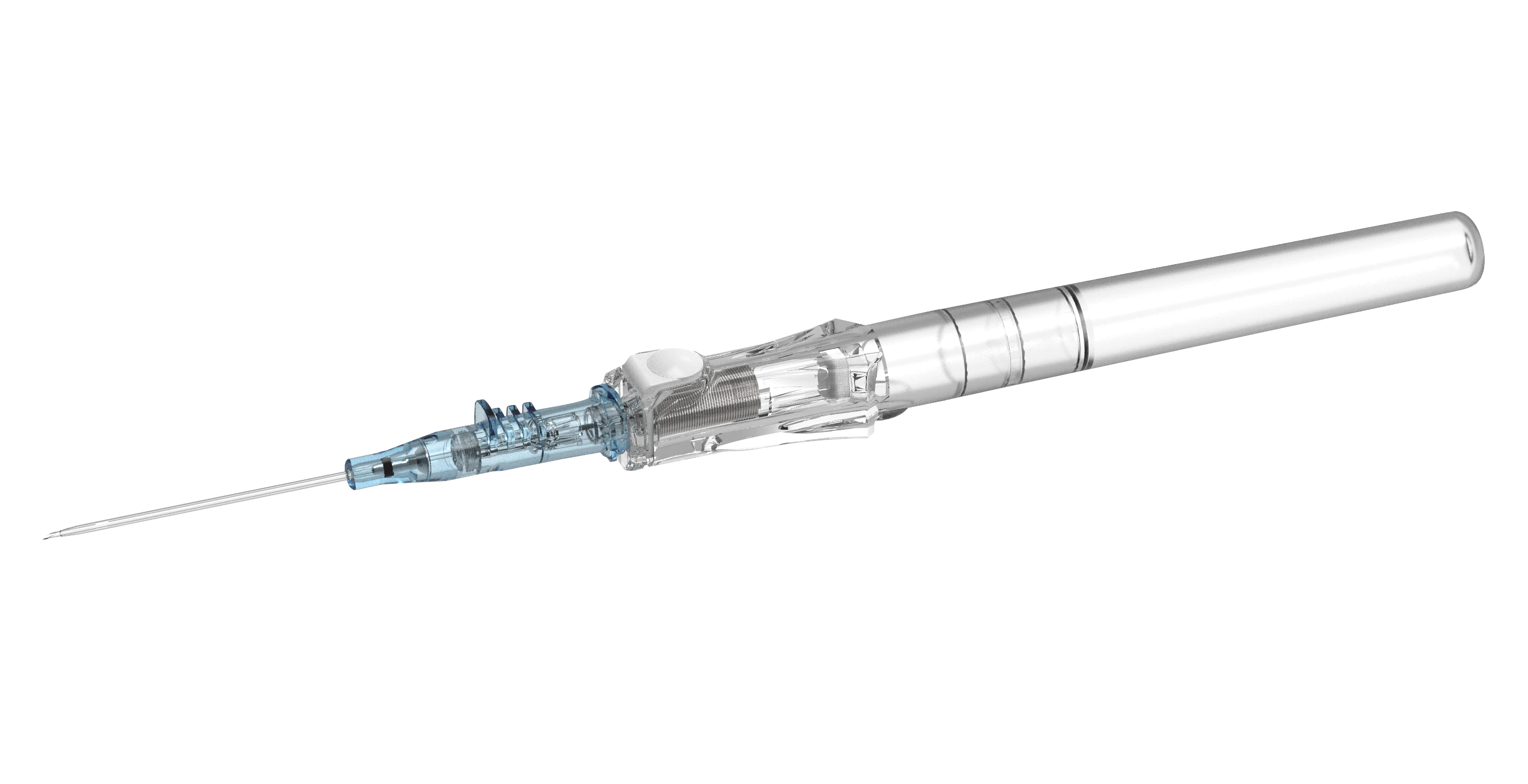
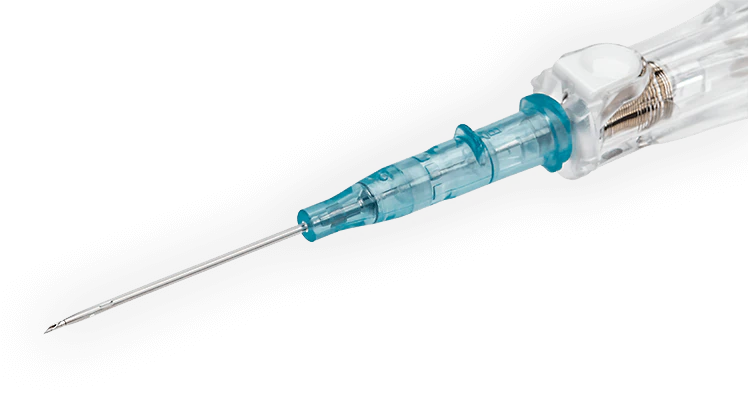
BD インサイト™ オートガード™ BC Pro (逆流防止機能付き)針刺し損傷防止機構付き静脈留置カテーテルは、プッシュボタン式ニードルシールド技術の搭載により、ボタンを押すとセーフティバレル内に内針が瞬時に収納され、針刺し損傷のリスクを低減します。また、初回挿入成功率を高める BD インスタフラッシュ™ ニードル技術を採用。素材に使用されている BD バイアロン™ カテーテルは、静脈内で最大 70% 柔らかくなることで長期留置を可能とし、血管損傷のリスクが低減されることが報告されています 2+ 。

- 概要
- 製品・アクセサリ
- 関連情報
圧迫止血が不要
内針を抜き取った際、圧迫止血を行わなくても血液の逆流がカテーテル内で止まる逆流防止機能を有します1。
血液曝露のリスク低減
静脈血管を穿刺する際には、血液曝露のリスクがともないます。
BD インサイト™ オートガード™ BC Pro(逆流防止機能付き)は血液曝露のリスクを低減する血液逆流防止機能と針刺し損傷防止機能を有しています。
針刺し損傷のリスク低減
プッシュボタン式針完全収納タイプは針刺し損傷のリスクを低減すると報告されています3。
患者さんのケアを第一優先に
血液漏れが少なくなるため、患者さんのケアに集中することができます。
販売名:BD インサイト オートガード
医療機器認証番号:20500BZY00636000
製造販売元:日本ベクトン・ディッキンソン株式会社
† Compared to an FEP catheter.
1. Onia R, Eshun-Wilson I, Arce C, et al. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. Curr Med Res Opin. 2011;27(7):1339-1346.
2. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Ann Intern Med. 1991;114:845-854.
3. Mendelson MH, Lin-Chen BY, Finkelstein-Blond L, Bailey E, Kogan G. Evaluation of a safety IV catheter (IVC) (Becton Dickinson, Insyte Autoguard): final report [abstract]. Eleventh Annual Scientific Meeting Society for Healthcare Epidemiology of America. 2001.