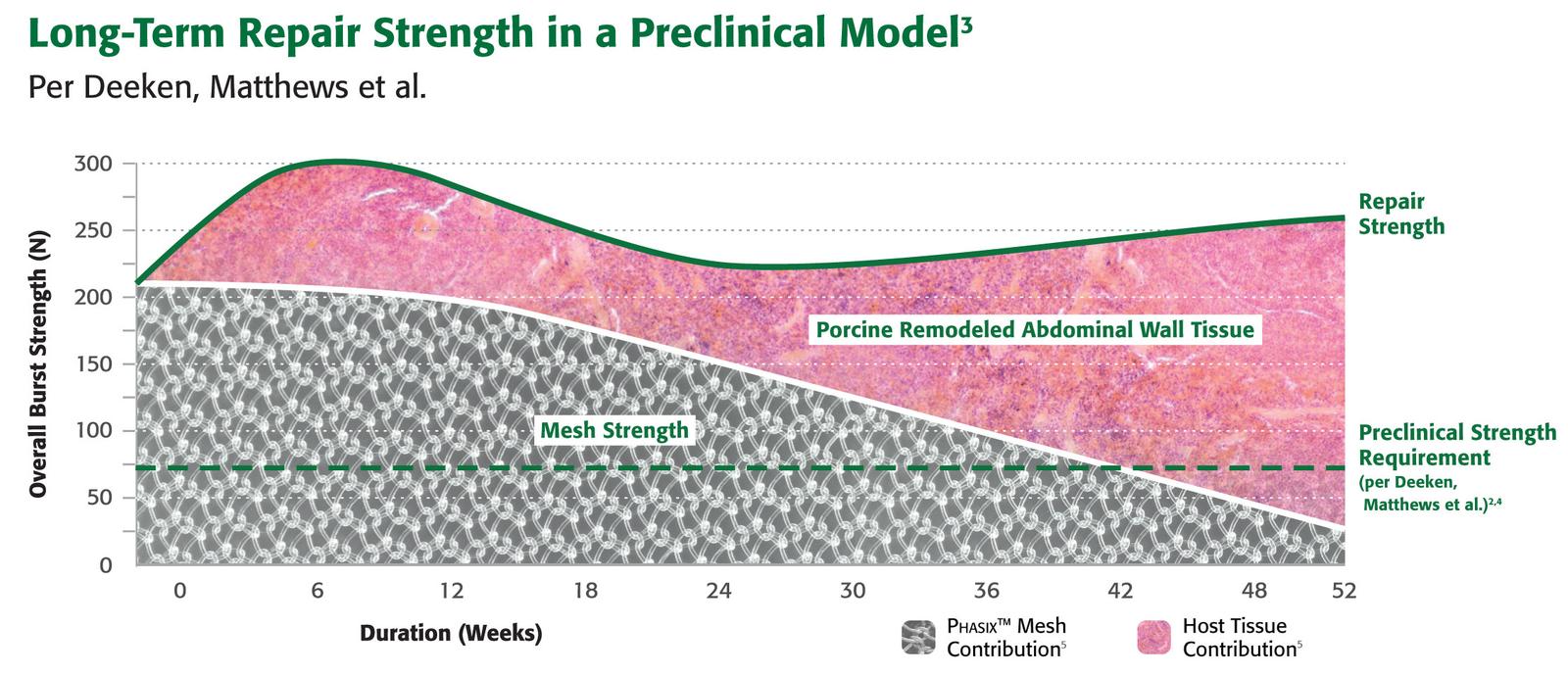
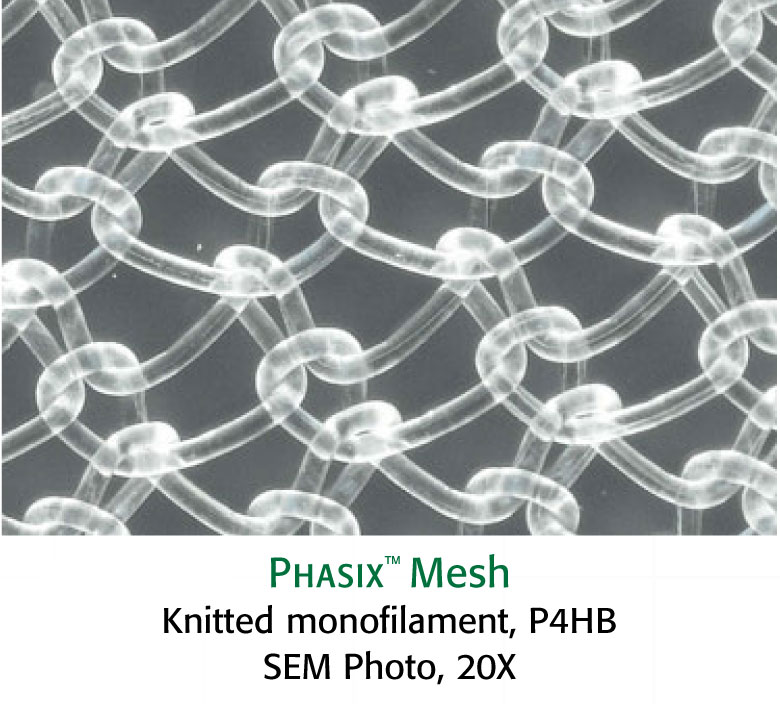
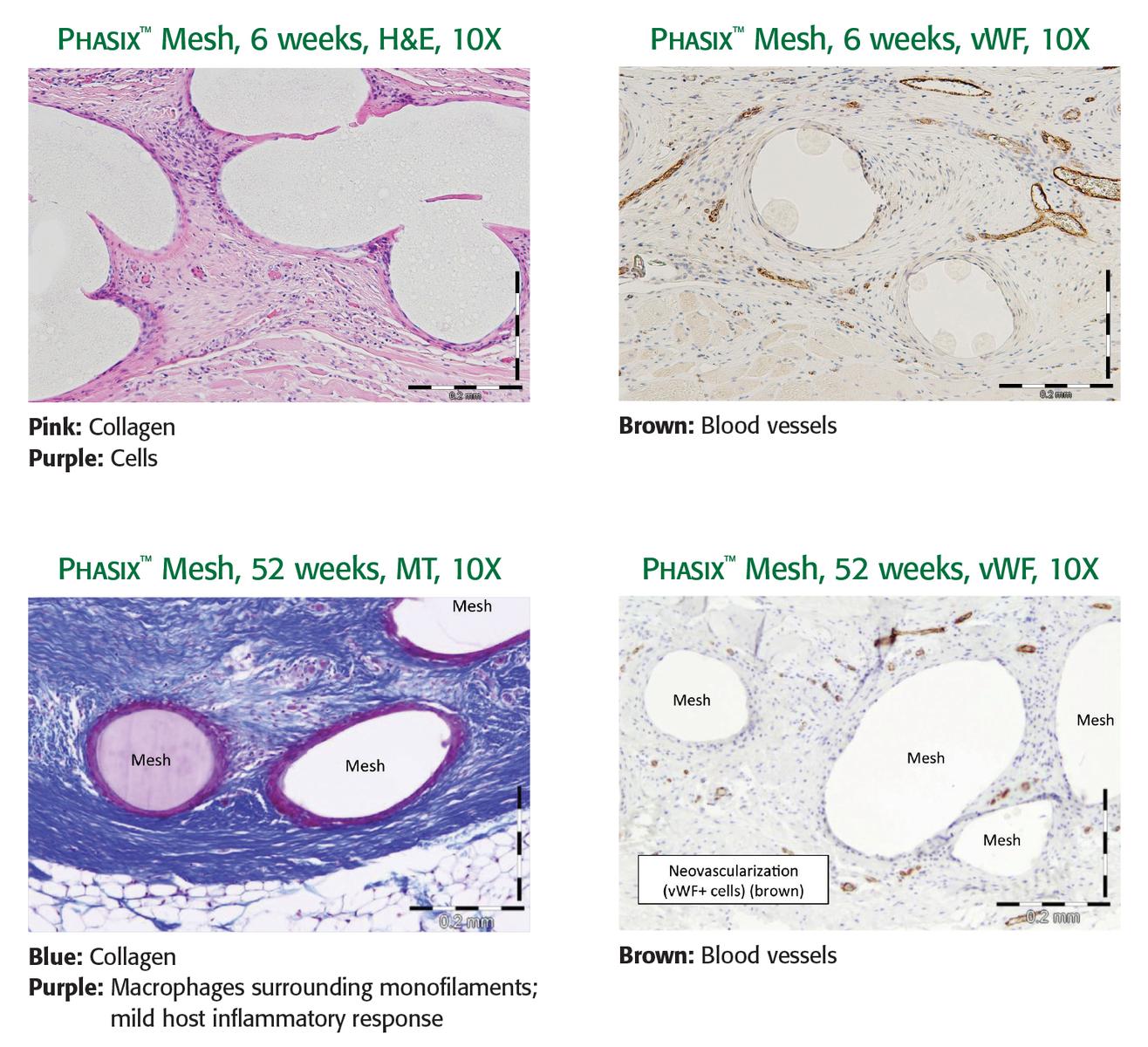
Preclinical data on file. Results may not correlate to clinical performance in humans.
Deeken CR, Abdo MS, Frisella MM, Matthews BD. “Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair.” Surgical Endoscopy 25.5 (2010): 1541-552.
Estimated from Standard Curve in (Martin DP, et al. “Characterizationof poly-4-hydroxybutyrate mesh for hernia repair applications.” Journal of Surgical Research. 2013; (184): 766-773
Martin, DP. et al. “ Medical applications of poly-4-hydroxybutyrate:a strong flexible absorbable biomaterial. Biochemical Engineering Journal. 2002; December 9
Amid PK, Shulman AG, Lichtenstein IL, Hakaha M. Biomaterials for Abdominal Wall Hernia Surgery and Principles of their Applications. Langenbecks Archive Chir. 1994; 379(3): 168-71.
Halaweish I, Harth K, Broome AM, Voskerician G, Jacobs MR, Rosen M. Novel In Vitro Model for Assessing Susceptibility of Synthetic Hernia Repair Meshes to Staphylococcus aureus Infection Using Green Fluorescent Protein-Labeled Bacteria and Modern Imaging Techniques. J Surg Infect (Larchmt). 2010; Oct1(5): 449-54.
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Phasix™ Mesh is indicated to reinforce soft tissue where weakness exists, in patients undergoing abdominal, plastic, and reconstructive surgery in ventral hernia repair and other abdominal fascial defect procedures.
Contraindications
Because Phasix™ Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Phasix™ Mesh must not be put in direct contact with the bowel or viscera.
The use of any mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh.
Mesh manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. The use of this mesh in susceptible patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
The safety and effectiveness of Phasix™ Mesh in the following applications has not been evaluated or established: a. Pregnant or breastfeeding women b. Pediatric use c. Neural and cardiovascular tissue.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require the removal of the mesh.
To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
The mesh is supplied sterile. Prior to use, carefully examine package and product to verify neither is damaged and that all seals are intact. Do not use if the foil pouch or package is damaged or open, or if the center of the temperature indicator on the foil pouch is black.
This mesh has been designed for single use only. Reuse, reprocessing, resterilization, or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness, or death of the patient or end user.
If unused Phasix™ Mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, handle and dispose of in accordance with accepted medical practice and applicable local, state, and federal laws and regulations to prevent risk of transmission of viral infections.
This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
This mesh is not for the use of treatment of stress urinary incontinence.
Phasix™ Mesh has not been studied for use in breast reconstructive surgeries.
Precautions
Please read all instructions prior to use.
Only physicians qualified in the appropriate surgical techniques should use this mesh. Users should be familiar with mesh strength and size requirements. Improper selection, placement, positioning, and fixation of the mesh can cause subsequent undesirable results.
Clinical data in accordance with EU MDR has not been established for laparoscopic/robotic procedures.
The safety and effectiveness of Phasix™ Mesh in the proximity of existing or excised cancer has not been established.
Adverse Reactions.
In preclinical testing, Phasix™ Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to infection, seroma, pain, mesh migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, allergic reaction, extrusion, erosion, fistula formation and recurrence of the hernia or soft tissue defect. Please consult product package insert for more detailed safety information and instructions for use.