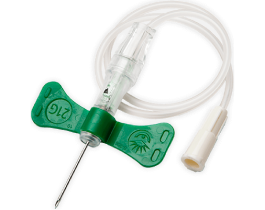
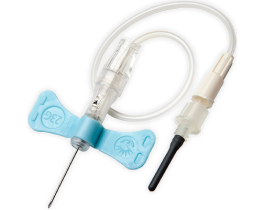
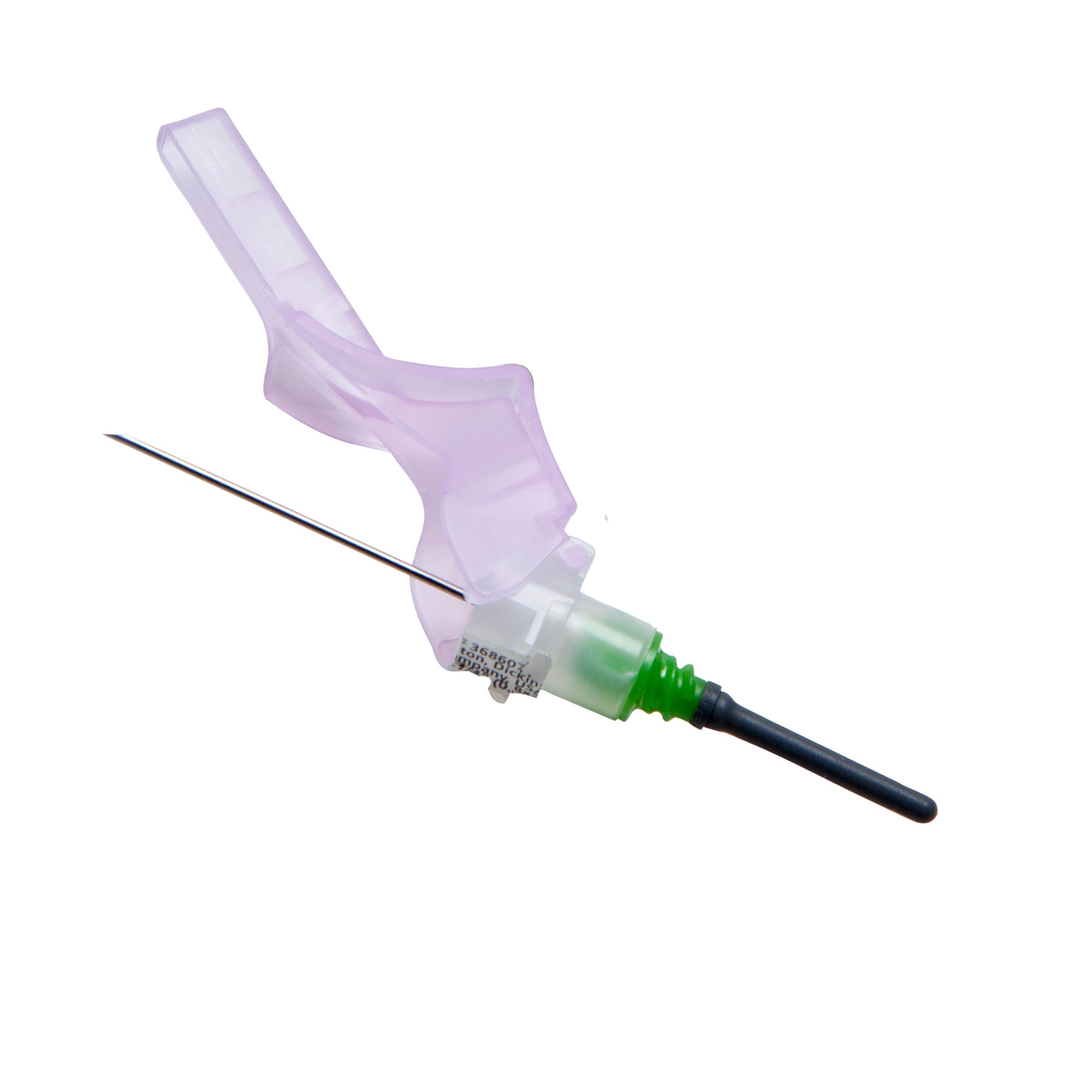
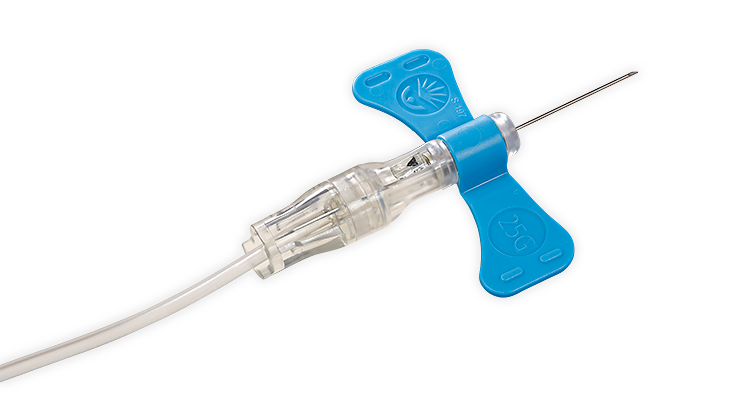
The BD Vacutainer® Push Button Blood Collection Set features a push button safety mechanism that instantly helps protect you against needlestick injury. Its in-vein activation reduces the risk of healthcare worker exposure to a contaminated needle, provides easy activation without patient discomfort and works well in high-risk environments.
It is also offered with a pre-attached holder to add convenience.
The BD Vacutainer® Push Button Blood Collection Set with Pre-Attached Holder can help guard against costly, potentially debilitating needlesticks, allowing you and your staff to focus on delivering superior patient care.