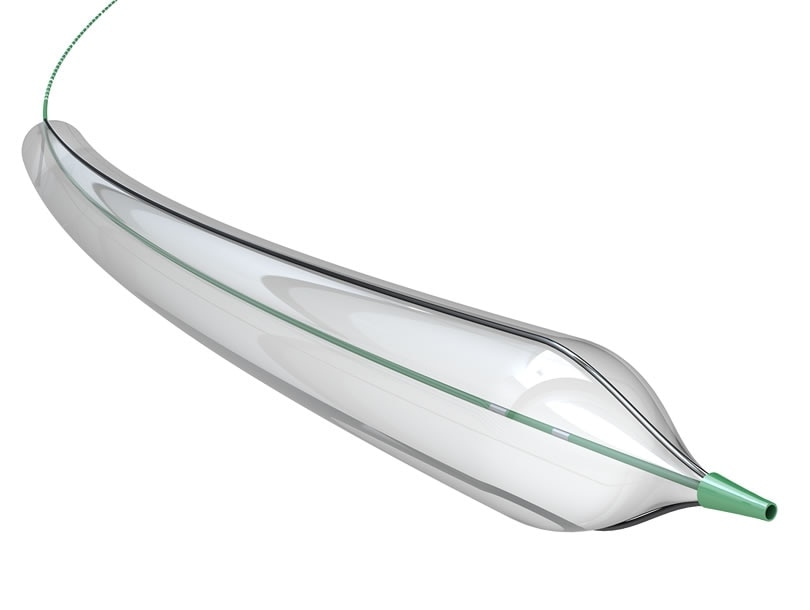
The UltraScore™ Balloon is the first commercially available .035” scoring balloon, with sizes available on both .014” and .035” guidewire platforms.

- Overview
- Products & Accessories
- EIFU & Resources
Features
- Specialty Scoring Balloon
- Focused Force Dilatation
- Low Profile, Over-the-Wire Design
- 2 Longitudinal .010″ wires placed 180 degrees apart
- .014″ Compatible
- Broadest Scoring Balloon Portfolio (up to 300mm)2
- GeoAlign™ Marking System
Compared to a standard PTA balloon of the same size, UltraScore™:
- Provides Approximately 24 times more force where the wire contracts the lesion1
- Is designed to longitudinally fracture plaque at lower inflation pressure
- May allow for more controlled plaque fracture and less vessel recoil, even in calcified lesions
UltraScore™ Focused Force PTA Balloon is available on a .014″platform, and is the lowest profile scoring balloon on the U.S. market.2
- Only 4F scoring balloon on the U.S. market2
- First .035″ scoring balloon commercially available on the U.S. market2
- Hydrophilic coating for optimized deliverability on .014” platform
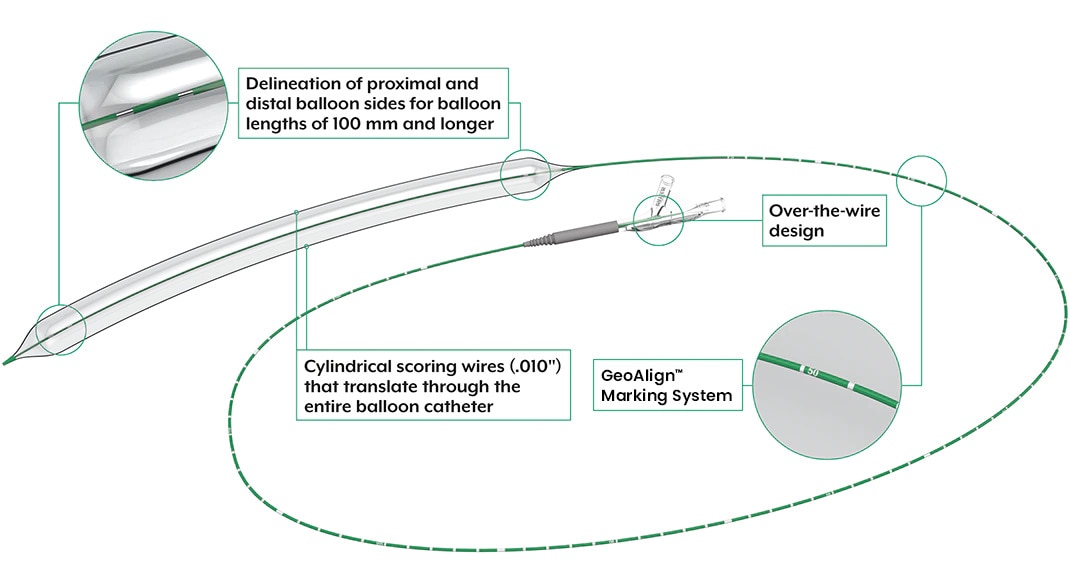
The UltraScore™ Focused Force PTA Balloon is the first scoring balloon to feature the GeoAlign™ Marking System for use as an intravascular measurement tool
- Designed to reduce fluoroscopy time, up to 27% in a pre-clinical study3
- Simple-to-use-, non-radiopaque ruler on the catheter shaft is designed to facilitate repeat catheter and geographic alignment3
Broadest Scoring Balloon Portfolio on the U.S. Market2
Broadest Size Matrix
- First .035" scoring balloon commercially available on the U.S. market
- More size offerings on an .014" and .035" wire4
- Hydrophilic coating for optimized deliverability on .014" platform
- Only 4F scoring balloon on the U.S. market2
.035" | |
| Shaft Lengths (cm) | 130 |
| Balloon Diameters (mm)5 | 4, 5, 6, 7, 8 |
| Balloon Lengths (mm) | 20, 40, 80, 100, 120, 150, 200, 300 |
| Shaft Compatibility (F) | 5,6 |
.014" | |
| Shaft Lengths (cm) | 150 |
| Balloon Diameters (mm)5 | 2, 2.5, 3, 4, 5, 6, 7 |
| Balloon Lengths (mm) | 40, 100,150, 300 |
| Shaft Compatibility (F) | 4,5 |
1. Based on theoretical calculation using equation P=F/A comparing UltraScore™ Balloon to POBA. Data on File, Bard Peripheral Vascular, Inc., Tempe, AZ. May not be predictive of clinical performance. Different tests may yield different results.
2. As of January 2022.
3. When the catheter is introduced to the vascular system, the location of balloon should be confirmed while under fluoroscopic observation. GeoAlign™ Markers are not a replacement for fluoroscopy. Animal study (repeat PTA in swine artery) was performed by 3 physicians who tested the Lutonix™ 035 (no drug) and the Ultraverse® 035 pta Catheter, both with GeoAlign™ Markers, to POBA with no GeoAlign™ Markers(n=112, test n=96 (average placement time of 66 seconds), control n=16 (average placement of 90 seconds)). Animal data on file, Bard Peripheral Vascular, Inc. Tempe, AZ. Animal test results may not be indicative of clinical performance. Different test methods may yield different results.
4. Compared to all other scoring balloons on the U.S. Market
5. Not all diameters available in every length
Please consult Instructions for Use under Resources for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
BD-23452v3

