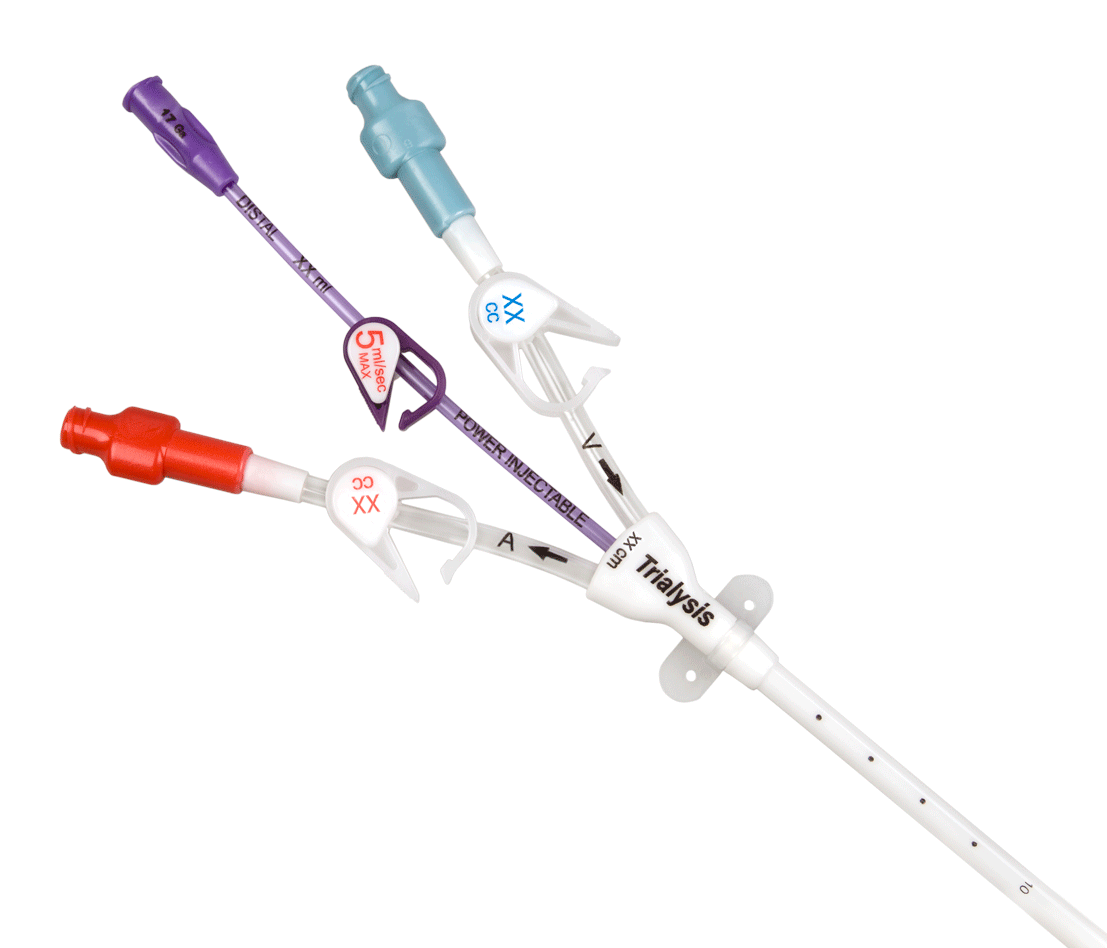
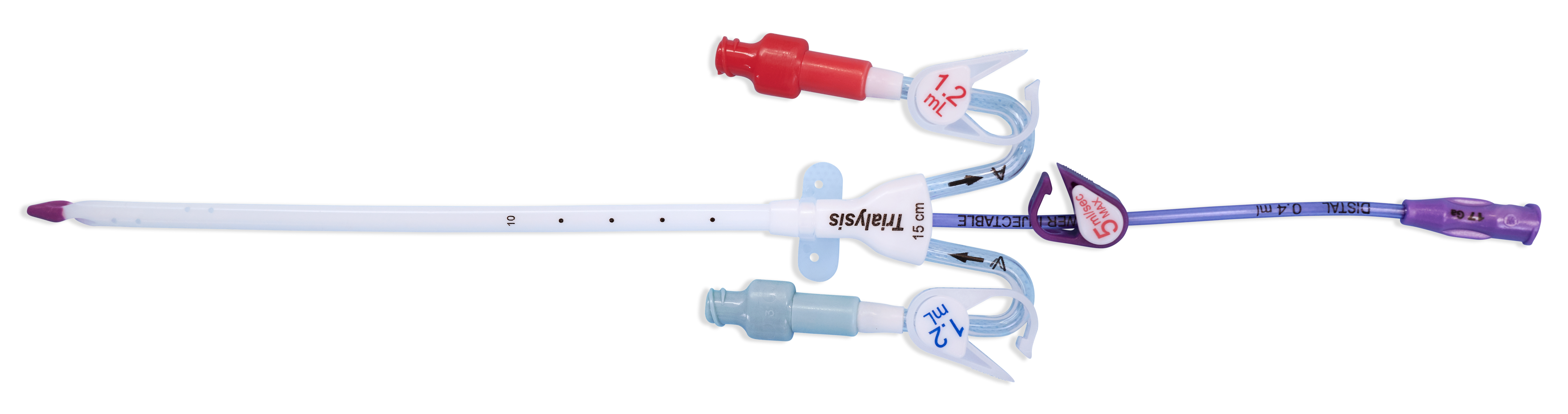
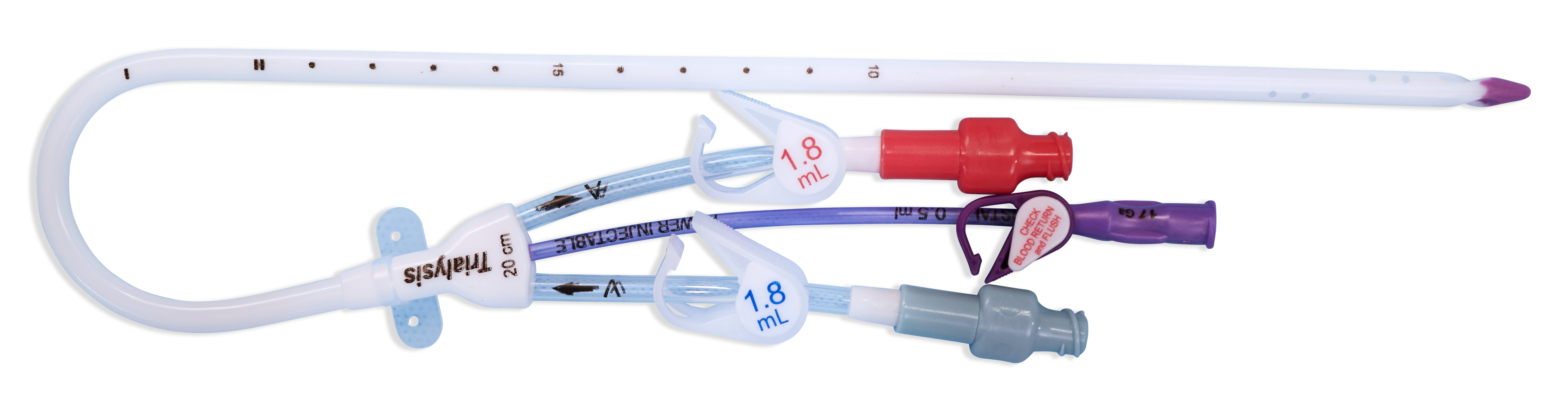
When your patient's therapy needs include a central line along with acute dialysis treatment, choose the Power-Trialysis™ Catheter. It is indicated for use, with a third internal lumen for intravenous therapy, power injection of contrast media, and central venous pressure monitoring, is indicated for use in attaining short-term (less than 30 days) vascular access for hemodialysis, hemoperfusion, and apheresis treatments. The catheter is intended to be inserted in the jugular, femoral, or subclavian vein as required. The maximum recommended infusion rate is 5 mL/sec for power injection of contrast media.
In today's environment, reducing exposure to bloodborne pathogens and bacteria to the clinician and the patient is extremely important. Having a central line integrated within an acute dialysis catheter may reduce clinician exposure with fewer dressings to maintain and may help improve patient experience by having fewer vascular access sites.
The Power-Trialysis™ Catheter family is made up of 12 and 13 Fr triple-lumen, short-term dialysis catheters that come in various lengths and configurations.
All Power-Trialysis™ Catheters are offered in standard kits as well as maximal barrier precaution trays.