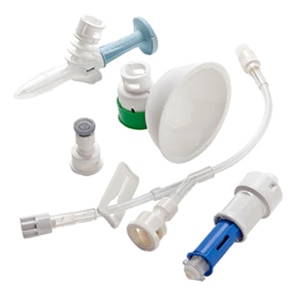
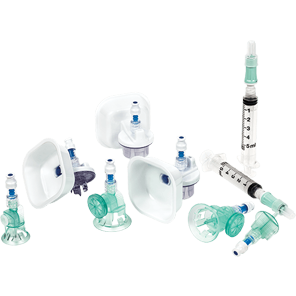
BD PhaSeal™ Optima system
The next-generation Closed-System Drug-Transfer Device (CSTD) from BD that advances hazardous drug protection
- Overview
- EIFU & Resources
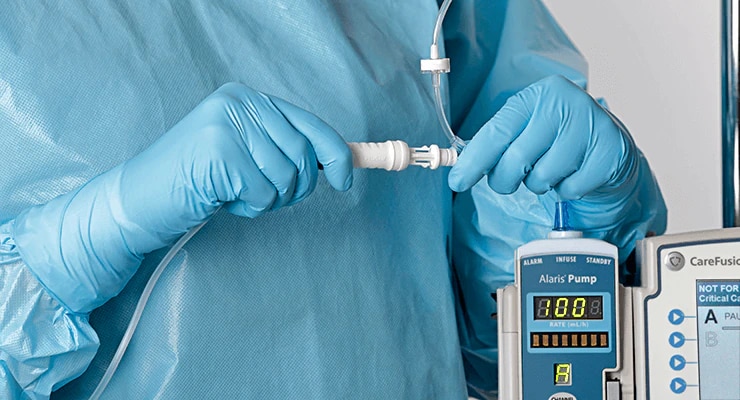
Safety. Performance. Ergonomics. Ease of use. We've optimized every component, and the result is a CSTD with design innovations that advance hazardous drug safety. The BD PhaSeal™ Optima system meets the requirements of the NIOSH IPA draft CSTD-test protocol‡, a robust test protocol applicable to barrier-type closed system drug transfer devices.1,2
Airtight
Every component is designed with no inlets or air exchange for completely airtight hazardous drug transfers.
Demonstrated
The BD PhaSeal™ Optima system prevents microbial ingress for up to 168 hours and 10 penetrations*†
Efficient
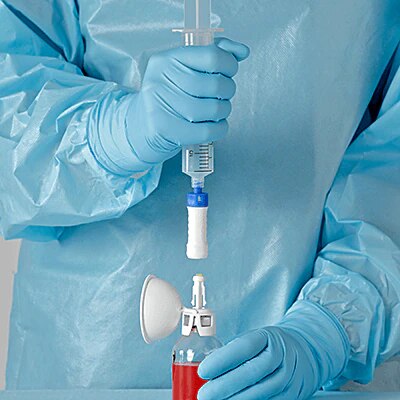
The BD PhaSeal™ Optima system minimizes residual drug loss in vials compared to other similar system.
User-validated
Feedback from longstanding CSTD users and traditional technique users guided us to optimize and innovate the design.
Safety
- Injector designed to prevent accidental needlesticks
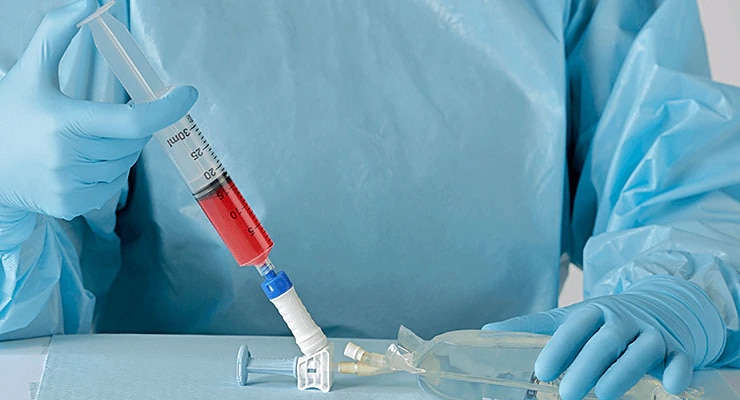
- Self-sealing membranes for leakproof connections
Performance
- Proprietary Protector design to maximize drug extraction
- Compatible with ISO-compliant Luer lock syringes and IV sets
Ergonomics
- Power grip and straight motion to connect and disconnect, aligned with NIOSH recommendations to avoid pinch grip3
- Optimized design for clinician comfort
Ease of use
- Intuitive one-step straight-push connection to aid workflow
- Can connect without alignment to help minimize learning curve
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD PhaSeal™ Optima Video for Pharmacy
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD PhaSeal™ Optima for Nursing
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes
‡2015
*Within an ISO Class V environment following aseptic technique.
†The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer’s recommendations and USP compounding guidelines for shelf life and sterility information.
References
- National Institute for Occupational Safety and Health (NIOSH). A vapor containment performance protocol for closed system transfer devices used during pharmacy compounding and administration of hazardous drugs. https://www.cdc.gov/niosh/docket/review/docket288/pdfs/a-vapor-containment-performance-protocol-for-closed-system-transfer-devices.pdf. Accessed May 26, 2020.
- BD-8440 BD PhaSeal Optima Vapor Containment White Paper.
- Easy Ergonomics: A Guide to Selecting Non-Powered Hand Tools. Centers for Disease Control and Prevention website. https://www.cdc.gov/niosh/docs/2004-164/pdfs/2004-164.pdf. Published August 18, 2004. Accessed April 22, 2020.
BD-20816