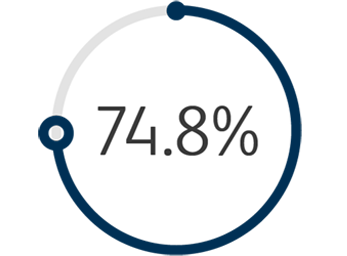
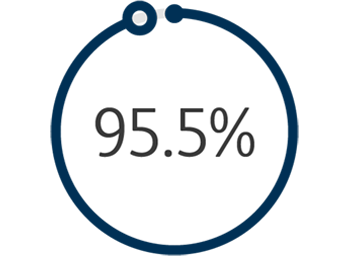
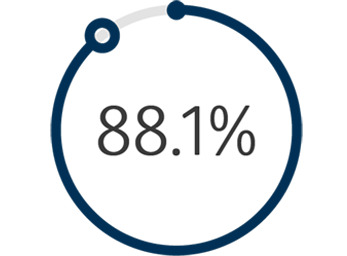
1The Venovo™ Venous Stent System was studied in the global VERNACULAR clinical trial, which was a prospective, multi-center, non-randomized, single-arm study of 170 patients. The primary effectiveness endpoint of the study was primary patency (PP) at 12 months post-index procedure, defined as: freedom from TVR and freedom from thrombus occlusion and stenosis > 50% as measured by DUS. Patients who received a Venovo™ Venous Stent had a weighted PP rate of 88.6%, demonstrating a statistically significant difference from a literature-derived performance goal (PG) of 74%, with an 81.7% PP rate for subjects with post-thrombotic syndrome and 97.1% PP rate for subjects with non-thrombotic iliac vein lesions. The primary safety endpoint was freedom from major adverse events (MAE) through 30 days post-index procedure. Freedom from MAE was 93.5%, demonstrating a statistically significant difference from a literature-derived PG of 89%. There were no stent migrations associated with CEC-adjudicated events at the 30-day primary safety endpoint or through 36 months. Primary Patency by Kaplan-Meier estimates at 36 months are 84.0% for the total population (N=170), 74.8% for subjects with post-thrombotic syndrome (N=93) and 95.5% for subjects with non-thrombotic iliac vein lesions. Secondary endpoints included acute technical success, Quality of Life (QoL) assessment, Venous Clinical Severity Score (VCSS – Pain score) and stent fractures. Results demonstrated 100% acute technical success, defined as successful deployment of stent(s) to intended target with adequate lesion coverage as assessed by the Investigator at the time of the index procedure. TLR is defined as the first revascularization procedure in the target vessel(s) following the index procedure, as determined by an Independent Core Lab. The Freedom from TLR rate at 36 months was 88.1%. At the 36-month follow-up, the CIVIQ-20 assessment demonstrated a change from baseline in the total study population of -16.8 and, for the VCSS Pain score, a change from baseline in the total population of -1.8. Stents were evaluated at the 36-month follow-up for fracture analysis. An anteroposterior and lateral x-ray for each evaluated stent were sent to an independent core lab for analysis. 98 subjects’ x-rays were analyzed and no stent fractures were reported. Missing x-ray analyses were recorded as protocol deviations. Dake, Michael D, et al. “Three-Year Results from the Venovo Venous Stent Study for the Treatment of Iliac and Femoral Vein Obstruction.” Cardiovasc Intervent Radiol, vol. 44, no. 12, Dec. 2021, https://doi.org/10.1007/s00270-021- 02975-2. Epub 2021 Sep 20
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, potential complications, adverse events and detailed safety information.