
- Overview
- Features
- Clinical Data
- EIFU & Resources
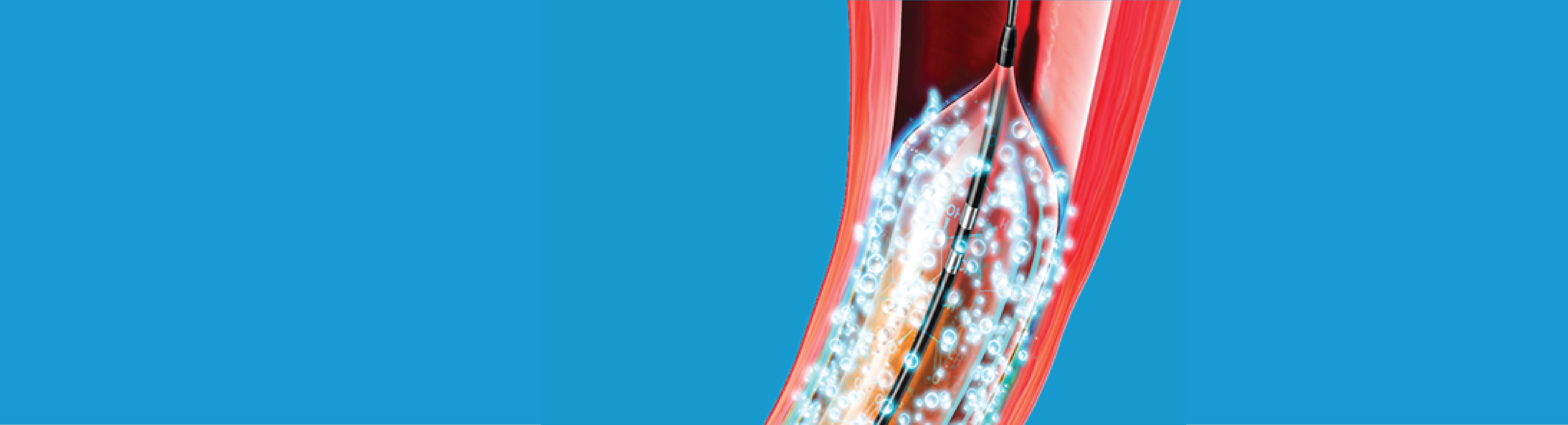
The Lutonix™ Drug Coated Balloon PTA Catheter is offered in 0.014” and 0.035” guidewire configurations for the treatment of peripheral arterial disease in the superficial femoral and popliteal arteries. The Lutonix™ DCB delivers a dose of 2 µg/mm2 of paclitaxel and is designed to provide the versatility needed to treat lesions of varying sizes and lengths. It is engineered to enhance femoropopliteal procedures by providing:
- A robust size matrix
- Extensive indications
- Proven results in a Level 1 Clinical Trial1
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
Low drug dose:
Optimal drug dose of 2 μg/mm2 of paclitaxel
Tapered tip:
Designed to facilitate advancement of the catheter to and through the stenotic region of the vessel.
Low sheath profile:
5F or less
Durable coating:
Designed to limit drug flaking during preparation and handling
Balloon lengths up to 300 mm on select 0.018 sizes
Only 300 mm DCB available in the U.S.2
100 cm and 130 cm catheter length:
To accommodate your preferred access site
0.018 and 0.035 guidewire compatibility:
To support your treatment algorithm
The GeoAlign™ Marking System is a standardized non-radiopaque ruler on the catheter shaft designed to:
- Facilitate repeat catheter placement
- Increase procedural efficiency
- LEVANT 2 clinical trial data on file. N=476. At 12 months, treatment with Lutonix™ 035 resulted in a primary patency rate of 73.5% versus 56.8% with PTA alone (p=0.001). Primary patency defined as absence of binary restenosis defined by DUS PSVR ≥ 2.5 and freedom from Target Lesion Revascularization (TLR). At 12 months, treatment with Lutonix™ 035 DCB resulted in a freedom from primary safety event rate of 86.8% versus 81.9% with PTA alone (p=0.185). Primary safety defined as composite of freedom from all-cause perioperative death and freedom at 1 year in the index limb from Amputation (ATK or BTK), Reintervention, and Index-limb related death. Numbers reported are Kaplan-Meier analyses, not pre-specified.
- As of May 2023. 300 mm only available on select 0.018 guidewire sizes.
- GeoAlign™ Markers are not a replacement for fluoroscopy. When the catheter is exposed to the vascular system, the location of the balloon should be confirmed while under high quality fluoroscopic observation. Animal study (repeat PTA in swine artery) was performed by 3 physicians who tested the Lutonix™ 035 DCB (no drug) and the Ultraverse™ 035 PTA Catheter, both with GeoAlign™ Markers, to POBA with no GeoAlign™ Markers (n=112, test n = 96, control n = 16). Animal data on file. BD Animal test results may not be indicative of clinical performance. Different test methods may yield different results.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information. ℞ only
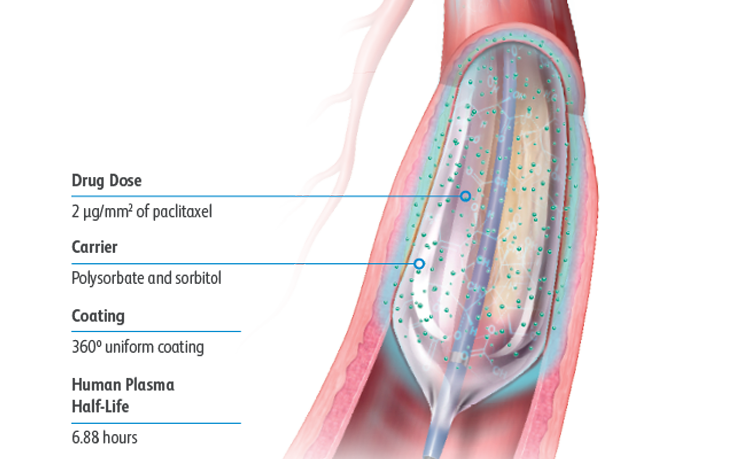
The Lutonix™ DCB formulation was designed for the optimal balance of safety and efficacy with a dose of 2 μg/mm2 and polysorbate and sorbitol excipients, which together allow for an effective transfer of drug to the vessel wall.
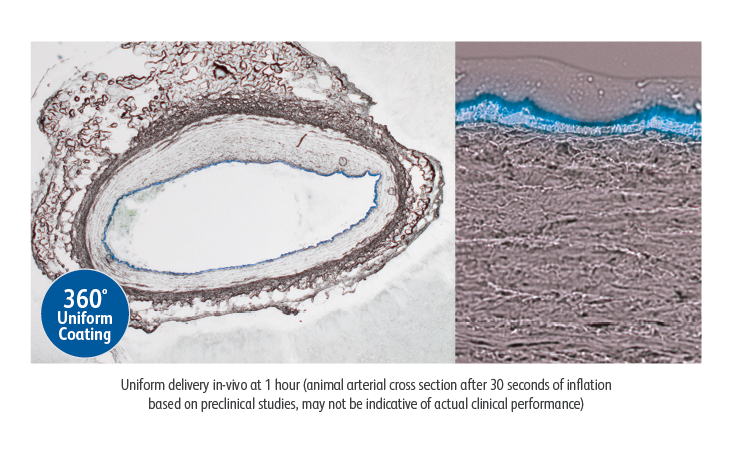
The Lutonix™ 035 DCB demonstrated a consistent coating resulting in:
- 360° of paclitaxel coating at the target vessel1
- ~6.46 μm coating thickness1

The Lutonix™ DCB coating formulation is designed to:
- Ensure rapid uptake of the drug to the vessel wall during inflation
- Provide uniform delivery of the drug to the vessel wall
- Help minimize unnecessary drug exposure to staff and patients
In a dry inflate shake test, the Lutonix™ 035 DCB showed a <0.1% drug loss2
Data from a Pre-Clinical Animal Study showed Lutonix™ DCB had desired pharmacologic levels with biologic effects without evidence of significant downstream emboli or systemic toxicity.3
1 Bench test and pre-clinical animal study data on file. Bench test results and preclinical data may not be indicative of clinical performance. Different test methods may yield different results.
2 Dry Inflate/Shake Bench Test data on file, BD, Tempe AZ. Shake test measured the average drug content lost after balloon was inflated, and after lightly knocking the device against the sides of a centrifuge tube, left and right, five times. n=5. Bench test results may not be indicative of clinical performance. Different test methods may yield different results.
3 Virmani Pre-Clinical Animal Data GLP Study. Pre-clinical data on file. Pre-clinical results may not be indicative of clinical performance. Different test methods may yield different results.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information. ℞ only
BD-38946
The Lutonix Global Registry showed sustained efficacy of Lutonix™ 035 DCB in heterogeneous patient population in real-world clinical practice.1
The primary efficacy endpoint was measured as Kaplan-Meier (KM) freedom full Target Lesion Revascularization and found to be 94.1% at 12 months and 90.3% at 24 months.1
The efficacy of the Lutonix™ 035 DCB was maintained in long lesions and in-stent restenosis subgroups with 88.2% and 84.6% freedom from TLR at 24 months, respectively.1
The LEVANT 2 pivotal study is a global, prospective, single-blind, randomized, 54-site study (42 sites in the United States and 12 in Europe) that enrolled all patients under one protocol, comparing the Lutonix™ 035 DCB with standard PTA.2
The study met its primary endpoints for safety and efficacy.
- Safety: Lutonix™ 035 demonstrated a safety profile that is consistent with PTA
- Efficacy: Lutonix™ 035 demonstrated a 29.4% better primary patency rate at 12 months than PTA
1 Lutonix Global SFA Real-World Registry, n=691. The primary safety endpoint was freedom from target vessel reintervention, major index limb amputation, and device- or procedure related death at 30 days. Freedom from primary safety events at 30 days was 99.4% (681/681). Primary efficacy endpoint is defined as freedom from TLR at 12 months. TLR-Free rate by subject counts at 12 months was 93.4% (605/648). The Kaplan-Meier TLR-Free survival estimate was 94.1% at 12 months and 90.3% at 24 months. All subjects treated with the Lutonix™ 035 DCB. In the LEVANT 2 IDE Clinical Trial, treatment with Lutonix™ 035 DCB resulted in freedom from TLR rate of 87.7% at 12 months (250/285) and a freedom from TLR rate of 82.0% at 24 months. Data on file, Becton, Dickinson and Company.
2 LEVANT 2 clinical trial data on file. N=476. At 12 months, treatment with Lutonix™ 035 resulted in a primary patency rate of 73.5% versus 56.8% with PTA alone (p=0.001). Primary patency defined as absence of binary restenosis defined by DUS PSVR ≥ 2.5 and freedom from Target Lesion Revascularization (TLR). At 12 months, treatment with Lutonix™ 035 DCB resulted in a freedom from primary safety event rate of 86.8% versus 81.9% with PTA alone (p=0.185). Primary safety defined as composite of freedom from all-cause perioperative death and freedom at 1 year in the index limb from Amputation (ATK or BTK), Reintervention, and Index-limb related death. Numbers reported are Kaplan-Meier analyses, not pre-specified.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information. ℞ only
BD-38946
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information. ℞ only
BD-38946
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information. ℞ only
BD-38946