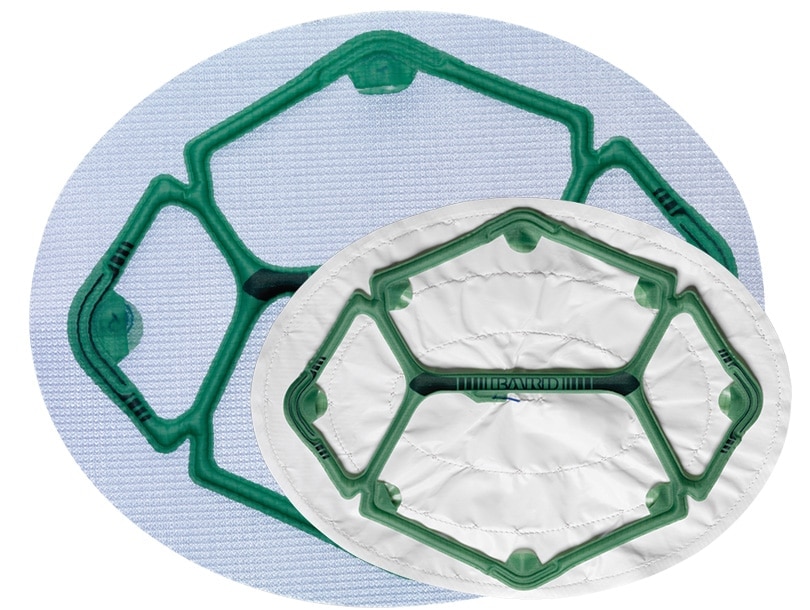
Ventralight™ ST Mesh or Composix™ L/P Mesh with a pre-attached low-profile balloon to help facilitate deployment, placement and positioning in laparoscopic ventral hernia repair.
Ventralight™ ST Mesh with Echo PS™ Positioning System, Ellipse, 6 in x 8 in (15.2 cm x 20.3 cm)
- Overview
- Specifications
- eIFUs and Resources
- FAQ
- Product Notifications
GTIN - Case
00801741201998
1
Quantity
1/cs
Dimensions
6” x 10” (15.2cm x 25.4cm) Oval
GTIN
| GTIN - Case | 00801741201998 | 1 |
Packaging
| Quantity | 1/cs |
Product Basic Specification
| Dimensions | 6” x 10” (15.2cm x 25.4cm) Oval |
If you are a patient or end user, you can contact us yourself, or you may have your caregiver or your physician do that for you. To help us process your
information quickly and effectively, please contact our customer complaints
team.
To better facilitate our investigation, please include the following information in your reporting:
- Product Name and/or Catalog Number
- Lot Number or Serial Number
- Any injuries and/or Harm?
- What is the issue you experienced?
- Is the actual sample or sample representative available? (If possible, please send affected sample)
- Contact name and phone number
1 When compared to positioning with transfascial sutures in a preclinical study.
Date on file at C. R. Bard, Inc. Results may not correlate to performance in humans.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias. Composix™ L/P Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias and chest wall defects. The Echo PS™ Positioning System is intended to be used to facilitate the delivery of soft tissue prostheses during laparoscopic hernia repair.
CONTRAINDICATIONS
Literature reports there is a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
Ventralight™ ST Mesh/Composix™ L/P Mesh is the only permanent implant component of the device. The inflation adapter and syringe are to be kept external to the patient and discarded after use. The Echo PS™ Positioning System (including the balloon, all connectors, and inflation tube) is to be removed from the patient and appropriately discarded as it is not part of the permanent implant.
The Echo PS™ Positioning System should not be used with any other hernia prosthesis aside from those with which it comes pre-attached/packaged.
PRECAUTIONS
Do not trim the mesh. This will affect the interface between the mesh and positioning system.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use
.
