BD-23530

- Overview
- Products & Accessories
- EIFU & Resources
- FAQ
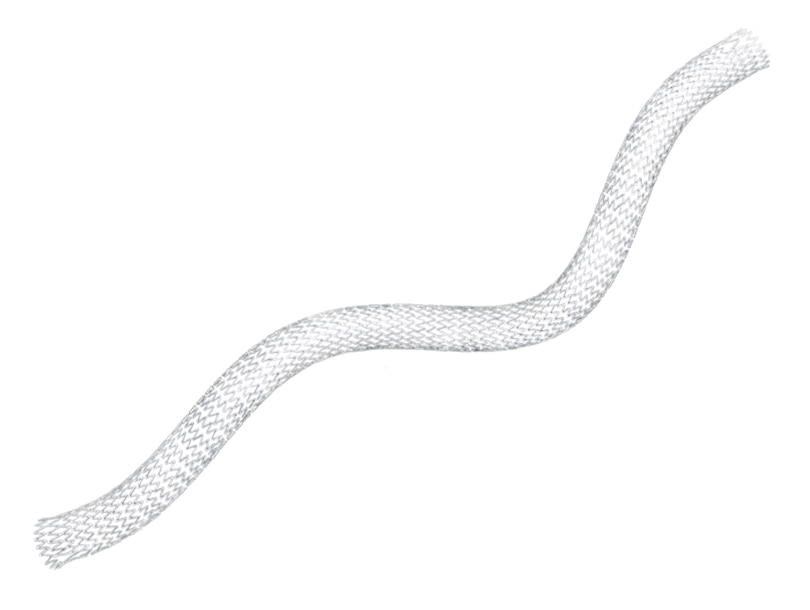
The LifeStent™ Vascular Stent is designed to deliver a self-expanding stent to the peripheral vasculature via a sheathed delivery system. With its unique helical design, it is engineered for bending, compression, and torsion with dynamic vessel conformability. The LifeStent™ Vascular Stent Systems, in varying sizes, have been studied in more than ten clinical trials in the United States and globally.
1. As of December 2020
2. Laird et al. Nitinol Stent Implantation vs. Balloon Angioplasty for Lesions in the Superficial Femoral and Proximal Popliteal Arteries of Patients With Claudication: Three-Year Follow-up From the RESILIENT Randomized Trial. J Endovasc Ther. 2012;19:1-9. The LifeStent® 5 mm diameter was not included in this trial.
3. RESILIENT I Trial, RESILIENT II Trial, E-TAGIUSS Trial, Retrospective Analysis of LifeStent® Vascular Stent Systems in the Treatment of Long-Segment Lesions, LifeStent® Vascular Stent Delivery System Study (LifeStent® 200 mm Trial),REALITY I Trial,REALITY II Trial, ETAP Trial, CONTINUUM Trial, and RELIABLE Trial. Additional trials ongoing.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
1. As of December 2020
2. Laird et al. Nitinol Stent Implantation vs. Balloon Angioplasty for Lesions in the Superficial Femoral and Proximal Popliteal Arteries of Patients With Claudication: Three-Year Follow-up From the RESILIENT Randomized Trial. J Endovasc Ther. 2012;19:1-9. The LifeStent® 5 mm diameter was not included in this trial.
3. RESILIENT I Trial, RESILIENT II Trial, E-TAGIUSS Trial, Retrospective Analysis of LifeStent® Vascular Stent Systems in the Treatment of Long-Segment Lesions, LifeStent® Vascular Stent Delivery System Study (LifeStent® 200 mm Trial),REALITY I Trial,REALITY II Trial, ETAP Trial, CONTINUUM Trial, and RELIABLE Trial. Additional trials ongoing.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1. As of December 2020
2. Laird et al. Nitinol Stent Implantation vs. Balloon Angioplasty for Lesions in the Superficial Femoral and Proximal Popliteal Arteries of Patients With Claudication: Three-Year Follow-up From the RESILIENT Randomized Trial. J Endovasc Ther. 2012;19:1-9. The LifeStent® 5 mm diameter was not included in this trial.
3. RESILIENT I Trial, RESILIENT II Trial, E-TAGIUSS Trial, Retrospective Analysis of LifeStent® Vascular Stent Systems in the Treatment of Long-Segment Lesions, LifeStent® Vascular Stent Delivery System Study (LifeStent® 200 mm Trial),REALITY I Trial,REALITY II Trial, ETAP Trial, CONTINUUM Trial, and RELIABLE Trial. Additional trials ongoing.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.